Disease Management
The snapshots on this page describe methods currently under development that could detect HLB early in the infection process (weeks or a few months, rather than years).
-
Role of salicylic acid in growing citrus under endemic HLB
Research by Drs. Lukasz Stelinski (University of Florida), Mamoudou Sétamou (Texas A&M), and Freddy Ibanez (University of Florida)
Article written by Freddy Ibanez and Lukasz Stelinski. Edited by Sara Garcia-Figuera, Peggy G. Lemaux, Monique Rivera, and Madison Sankovitz.
Revised July 8, 2025.What is the research?
In areas wherein HLB is endemic (nearly 100% of citrus trees are infected), slowing the spread of HLB is no longer needed. The main grower concern is producing a profitable yield under existing conditions. Replicated trials have proven that suppression of Asian citrus psyllid (ACP) populations causes a measurable boost in citrus yield, even when all trees are infected. Yet, the need for ACP management in areas where HLB is endemic is questioned, given the associated costs. In this research, we explore the interactions among the HLB bacterium, the ACP vector and sweet orange to determine a possible mechanism by which reducing vector populations benefits the health of citrus under endemic HLB conditions.
Plants defend themselves against attackers by activating their innate immunity system. Damage caused by phloem-feeding insects, such as ACP, and plant pathogenic bacteria are recognized by plants and trigger defense responses that are regulated by plant hormones, including salicylic acid (SA). The objective of this research was to describe the biological function of a set of genes associated with the synthesis of SA and its metabolites to determine how their accumulation in plant tissues regulates defense responses to both the HLB bacterium and damage caused by the ACP vector. The long-term goal of this research is to boost natural defenses of citrus against the HLB pathogen and ACP vector.
How does SA improve HLB management?
We found that in the absence of coincident ACP feeding damage, citrus trees could mount a defense response against the HLB bacterium by activating the SA pathway. Repe
 ated, monthly ‘pulses’ of ACP feeding led to pronounced stimulation of the cellular machinery that makes SA and this was coincident with lower bacterium titers in plants. However, continuous and/or long-term (≥ 270 d) ACP feeding shut down defense responses against the bacterium (Figure 1).
ated, monthly ‘pulses’ of ACP feeding led to pronounced stimulation of the cellular machinery that makes SA and this was coincident with lower bacterium titers in plants. However, continuous and/or long-term (≥ 270 d) ACP feeding shut down defense responses against the bacterium (Figure 1).Our results provide a mechanism explaining how vector suppression contributes to maintaining the health of cultivated citrus in areas where HLB is endemic. Also, specific gene targets were identified that may yield novel genotypes expressing tolerance against CLas after appropriate manipulations.
Who is working on the project?
Drs. Lukasz L. Stelinski, Freddy Ibanez, and Mamoudou Sétamou conceived, designed, and carried out this research. Stelinski and Ibanez are located at the University of Florida’s Citrus Research and Education Center in Lake Alfred, Florida. Dr. Setamou is with the Texas A&M Department of Entomology, and he is at the Kingsville Citrus Center in Kingsville, TX.
What are the challenges and opportunities?
Our results point to specific gene targets that may yield novel genotypes expressing tolerance against CLas. For example, decreasing expression of certain transcription cofactors (NPR3/NPR4) may enhance tolerance to CLas and is currently being explored.
Our results also provide a mechanism that explains how vector suppression contributes to the health of citrus cultivated in areas where HLB is endemic. Reducing stress caused by vector feeding allows trees to better defend themselves against the consequences of pathogen infection. Therefore, the cost of vector suppression is worth the investment, even when HLB is endemic. However, employing genetic engineering as a method to overcome HLB will depend on effectively informing consumers regarding the safety of such produce and their accepting that assurance.
Funding source: This work was supported by U.S. Department of Agriculture HLB Multi-Agency Coordination (MAC) system (AP17PPQS&T00C163) to L.S. and M.S.
-
Automated delivery system for therapeutic materials to treat HLB-infected citrus
Research by Dr. Ozgur Batuman, University of Florida
Article written by Drs. Ozgur Batuman and Louise Ferguson. Edited by Sara Garcia-Figuera, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, Monique Rivera, Madison Sankovitz, and Lukasz Stelinski.
Revised July 7, 2025.What is the technique?
The bacterium that causes huanglongbing (HLB), “Candidatus Liberibacter asiaticus” (CLas), enters a citrus tree either through grafts or when the Asian citrus psyllid (ACP) feeds. Once inside the tree, the bacterium proliferates in the phloem. The phloem is the portion of the plant vascular tissue that transports sugars from their site of production in the leaves to other plant parts, such as the roots or flowers, that need energy for growth. Phloem can transport sugars up and down the plant. Therefore, once the HLB bacterium is inside the phloem, it has the potential to infect the entire tree.
Thus far, no treatment has been developed to prevent HLB infection or control the bacterium once inside the tree. This is in part due to the difficulty of reaching the phloem through conventional methods. Methods such as foliar spraying or soil drenching are not efficient routes to achieve the needed concentrations. Many chemicals are being investigated, but in order to test them, an effective method of direct or indirect delivery into the phloem is needed.
What is the focus of this project?
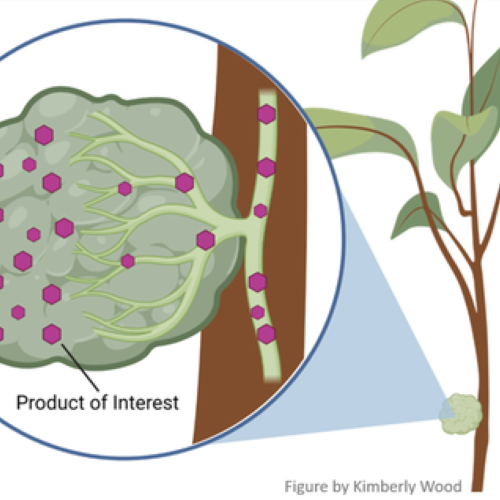
Our project focuses on developing an automated method for delivering therapeutic liquid materials containing bactericides, microbial metabolites, small RNAs, or biological control agents, into the citrus vascular tissues. This method could deliver the materials to the phloem, which conducts sugars and other metabolic products downward from the leaves, or to the xylem, which conducts water and nutrients upward from the roots to the leaves. Past delivery methods based on foliar sprays and root drenches have not been successful. We are investigating two alternative methods: 1) diffusion, in which the trunk is punctured and connected with a liquid reservoir, from where the liquid flows through passive uptake and is infused into the vascular tissue of the trunk, and 2) infusion, in which the trunk is actively injected with the liquid using a low-pressure application.
Who is working on the project?
The project is led by plant pathologist, Dr. Ozgur Batuman, with colleagues at the Southwest Florida Research and Education Center (SWFREC) at the University of Florida in Immokalee. In addition to the research described above, this four-year project will also study the citrus vascular system with a multidisciplinary research team, including UF Plant Pathologists Drs. Nabil Killiny and Amit Levy at Lake Alfred, SWFREC UF Plant Physiologist Ute Albrecht, Citrus Horticulturist Fernando Alferez, Precision Ag. Engineer Yiannis Ampatzidis, Agricultural and Natural Resources Economist Tara Wade, University of California-Davis Extension Specialist Louise Ferguson and Texas A&M-Kingsville Citrus Center Plant Pathologist Veronica Ancona as well as a number of graduate students, postdocs, and Florida, Texas and California citrus industry members.
How is the research being done?
Currently, the research is focused on optimizing the delivery method. Earlier research that compared delivery methods including foliar sprays, soil drenching, and trunk injection, determined that Needle-Assisted Trunk Infusion (NATI) was the best potential delivery method (Figure.1).
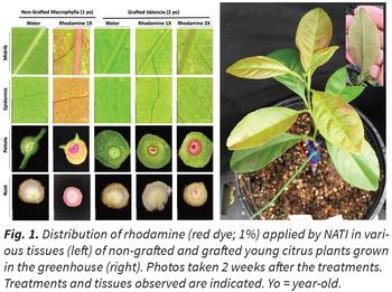
In initial experiments, a tracking dye, rhodamine (1%), was injected into the trunks of one-year-old citrus seedlings using NATI. A visible red color, indicative of rhodamine uptake and movement, was detected in the upper-most leaves within 30-60 min and an increase in color intensity was observed within 24 hours. Similar results were observed in two-year-old grafted Valencia plants within 48 hours.
If the NATI delivery method can be automated, large numbers of trees could be treated quickly. Our proposed automated delivery consists of a robotic arm with several modules at the end of the arm, installed on an all-terrain vehicle or tractor. One module with needles would grip and puncture the trunk, a second module would wrap a reservoir around the trunk below the punctures and a third module would fill the reservoir (Figure 2).
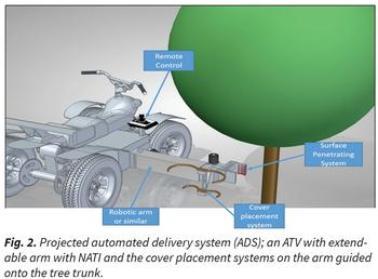
In addition to curing the targeted disease, automated delivery could aid disease prevention through the application of prophylactic chemicals that prevent infection. In this case, the system would be used to treat healthy young trees with bactericides or chemicals that boost their immune system. We envision the possibility of such treatments acting in an analogous fashion to vaccinations used to prevent diseases in humans and animals.
What are the challenges and opportunities?
The greatest challenge is successful, economical automated delivery of chemicals to the phloem. The greatest opportunity is the potential to develop a method that will allow much more precise delivery of treatments to citrus trees. In addition, this method could be used to control insects that feed on citrus plant parts; to deliver plant growth regulators, nutrients, and fertilizers, to roots and fruits to increase growth, development, and fruit quality; much like an intravenous injection functions in an animal.
For more information, please visit this project’s dedicated website:
https://swfrec.ifas.ufl.edu/programs/citrus-path/automated-delivery/ -
Progress toward HLB-tolerant citrus from conventional plant breeding
Research by Dr. Ed Stover, USDA-ARS, Fort Pierce, FL
Article written by Ed Stover, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Sara Garcia-Figuera.
Revised July 8, 2025What is the technique?
The USDA citrus breeding program has been underway since 1893 and numerous cultivated varieties have been released. USDA-produced varieties are represented in more than 75% of US- grown citrus trees. Like most fruit trees, citrus trees have a fruit-producing part (the scion), which is grafted onto a mostly underground part (the rootstock). The USDA team of Dr. Ed Stover focuses on developing new scions using conventional breeding approaches that involve crossing two complementary parents by transferring the pollen of one parent into the flowers of another parent. The resulting fruit produces seeds that, when mature, germinate and give unique new citrus types. The seedlings that result from crosses are then grown for a number of years until their fruit quality, productivity, disease resistance, and other traits can be assessed.
How does creating new varieties through breeding improve HLB management?
All Citrus cultivars tested so far are susceptible to huanglongbing (HLB), but they are not equally susceptible. Some are able to grow and produce a fairly normal crop, despite supporting high populations of the HLB-associated bacterial pathogen and developing leaf symptoms. The primary goal of the USDA scion-breeding program is to identify and develop HLB-tolerant citrus of major market types, such as mandarins, oranges, and grapefruit. Tolerance to HLB is measured by the ability of infected trees to produce sufficient numbers of attractive and flavorful fruit. That way, growers of such tolerant citrus varieties maintain profitable businesses and backyard growers are happy with their trees’ performance.
Currently, the most important goal for California citrus growers is preventing the disease from becoming established in the state. In Florida, despite using aggressive treatments to sustain HLB-affected trees, citrus production per acre has declined by ~40% and fruit quality has diminished. In addition, backyard citrus trees have essentially disappeared. In Florida, where HLB is endemic, tolerant citrus types will be immediately useful. In California, growers may choose to plant HLB-tolerant types as insurance against future losses, when HLB becomes established.
Who is working on the Project?
Citrus breeders and their research teams are collaborating with scientists from related disciplines throughout the U.S. to develop HLB-tolerant citrus. At the USDA/ARS in Florida, Ed Stover, Randy Driggers, Greg McCollum, David Hall, Liz Baldwin, Jinhe Bai and Anne Plotto collaborate to create, identify, and validate HLB-tolerant types producing high quality fruit. Colleagues at the University of California-Riverside, Mikeal Roose and Chandrika Ramadugu, and the University of Florida, Fred Gmitter, Ming Huang, and Qibin Yu, have collaborated with the USDA to identify gene markers associated with HLB-tolerance in USDA populations. These collaborations benefit from exchange of plant material, research results, techniques and ideas. UC Riverside and the University of Florida also have individual projects to develop HLB-tolerant citrus.
What are the challenges and opportunities?
The best sources of HLB tolerance include some mandarin types, citron, and the citrus relative Poncirus trifoliata. Unfortunately for Florida growers, the two most widely-grown mandarin varieties before HLB, Murcott and Sunburst, are highly susceptible and have been removed. A California mandarin (Tango), and a few minor Florida varieties (e.g. Sugar Belle and Bower), show some tolerance to bacterial strains present in Florida. A few advanced, high-quality mandarin hybrids in the USDA program have good HLB tolerance and are being evaluated for release.
 HLB tolerance associated with citron parentage is likely good news for lemon growers, but up until now, citron has been a parent only to acid citrus fruit types, like lemons and limes. We have initiated crosses between citron hybrids and other citrus types, like mandarins, oranges, and grapefruit. The USDA citrus breeding program has worked for over a century on hybrids between the foul-tasting Poncirus and the tasty Citrus types. Hybrids between Poncirus and Citrus have yielded many rootstocks over the years, and we have now developed more advanced hybrids that are acceptable scion types. The first such scion variety, ‘US SunDragon’, was recently released and it has shown remarkable tolerance to HLB. There is great promise for identification and development of HLB-tolerant citrus, but time and exposure to more diverse bacterial pathogen strains will be needed to prove their value.
HLB tolerance associated with citron parentage is likely good news for lemon growers, but up until now, citron has been a parent only to acid citrus fruit types, like lemons and limes. We have initiated crosses between citron hybrids and other citrus types, like mandarins, oranges, and grapefruit. The USDA citrus breeding program has worked for over a century on hybrids between the foul-tasting Poncirus and the tasty Citrus types. Hybrids between Poncirus and Citrus have yielded many rootstocks over the years, and we have now developed more advanced hybrids that are acceptable scion types. The first such scion variety, ‘US SunDragon’, was recently released and it has shown remarkable tolerance to HLB. There is great promise for identification and development of HLB-tolerant citrus, but time and exposure to more diverse bacterial pathogen strains will be needed to prove their value.Funding source: This project is funded by USDA/ARS base funds, the Citrus Research and Development Foundation, and the New Varieties Development and Management Corporation.
-
Controlling psyllid gut cell death to prevent Huanglongbing
Research by Dr. Michelle Cilia-Heck, USDA-Boyce Thomson Institute
Article written by Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 8, 2025What is the technique?
Plant pathogens can have devastating effects on plant health and can severely limit food production in agricultural crops. In the case of the devastating disease, huanglongbing (HLB), the tiny Asian citrus psyllid is the vector that picks up the bacterial pathogen (Candidatus liberibacter asiaticus - CLas) and transmits it to the next citrus tree during feeding. HLB is spread easily by psyllids. The focus of this research is determining if this transmission can be interrupted. Can the psyllid be prevented from picking up (acquiring) the pathogen when it feeds on an infected plant? And/or can the psyllid be prevented from transmitting the pathogen to a healthy plant?How does targeting cell death help?
The first step in this research is determining how CLas affects the Asian citrus psyllid vector. Plant pathogens transmitted by insects are not always benign ‘tag alongs’ in the vector. They can have negative or positive effects on vector health and function. Also, vectors often respond to the pathogens with defense mechanisms, even if they are not targets of infection. Cilia-Heck found that Asian citrus psyllids do respond to the CLas bacterium that causes HLB.
Cilia-Heck’s group found that CLas causes cell death, or apoptosis, in the gut cells of the psyllid vector. Apoptosis is a natural process of programmed cell death in multicellular organisms and its purpose is to get rid of unneeded or abnormal cells. Cells can initiate the process of cell death on their own, following stress or they can initiate the
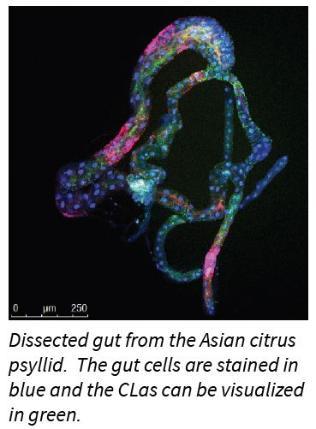
process when they receive signals from other cells. Though it’s a normal process, cell death can be shifted out of balance toward ‘too much or too little’ cell death and so affect the normal functions of the organism.Recent viral research suggests programmed cell death is a defensive mechanism that insects use to cope with viruses, which viruses in turn exploit to be successfully transmitted among hosts. The same may be taking place with the CLas - Asian citrus psyllid interaction. The psyllid’s gut cell death response may benefit the bacteria by allowing the bacteria to exit the gut and then enter the insects’ blood stream (hemolymph), ultimately reaching the insect’s mouthparts so that CLas can be passed on when the psyllid feeds on a new host plant.
Understanding how and why programmed cell death is triggered by CLas in the psyllid’s gut and whether this contributes to successful transmission among plants could have
important implications for managing HLB. If the bacterial proteins that bind to the gut of the Asian citrus psyllid could be identified, then methods could be developed to impede the CLas bacteria’s ability to bind to gut cells, cause cell death and move into the insect’s blood stream.Who is working on the Project?
Michelle Cilia-Heck, a USDA researcher at the Boyce Thomson Institute, and her research team are exploring various ways to manage HLB by disrupting transmission by psyllids.For more information see: Ghanim, M. et al. Morphological abnormalities and cell death in the Asian citrus psyllid (Diaphorina citri) midgut associated with Candidatus Liberibacter asiaticus. Sci. Rep. 6, 33418; doi: 10.1038/srep33418 (2016).
What are the challenges and opportunities?
Practical application of this new discovery requires finding specific proteins that bind to the gut and methods to alter or ‘silence’ them so that the bacteria can not bind to them, cause cell death and make their way into the blood stream of the insect. Development of this approach is estimated to take 3-5 years.Funding source: Controlling psyllid gut cell death to prevent Huanglongbing. The Science for Citrus Health project is funded by two grants from United States Department of Agriculture’s National
Institute of Food and Agriculture.
Designed by Barbara Alonso, University of California, Berkeley. -
Using genome editing to develop HLB-resistant or -tolerant citrus
Research by Drs. Wenbo Ma, Gitta Coaker, Nian Wang, Veronica Ancona and Georgios Vidalakis, University of California, Riverside
Article written by Wenbo Ma, Gitta Coaker, Nian Wang, Veronica Ancona, Georgios Vidalakis, Sara Garcia Figuera, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised June 27, 2025.What is the technique?
Scientists are studying the interaction at the molecular level between citrus trees and the bacterium Candidatus Liberibacter asiaticus (CLas) associated with huanglongbing (HLB). In particular, they are investigating how CLas interacts with citrus proteins to cause disease, hoping to disrupt this interaction and ultimately develop new resistant or tolerant varieties using a genome-editing technology called CRISPR/Cas.
What knowledge about HLB is needed to succeed in this strategy?
The first step undertaken by this team of scientists was to identify bacterial genes necessary for infection. Genes are sequences of deoxyribonucleic acid (DNA) that contain messages that code for the synthesis of proteins, which are molecules required for life processes in all organisms. Bacterial pathogens such as CLas secrete virulence proteins, called effectors, which enhance disease development by overcoming citrus defenses. Gitta Coaker’s group has been sequencing the genomes (the entire genetic information in an organism) of different strains of CLas to identify DNA sequences that could code for effectors. Effectors that are common to all strains of CLas found in different geographic areas are likely to be essential for the infection process, and therefore good targets for intervention. Wenbo Ma’s group is identifying the citrus proteins and biological processes that are targeted by these CLas effectors. In a complementary approach, Nian Wang has been using a genome-editing technology, the CRISPR/Cas system, to delete and modify citrus DNA sequences in order to change the citrus proteins that are targeted by the bacterial effectors, so they are no longer be susceptible to the effectors. Finally, Lisa House’s group is conducting customer and grower surveys to gauge acceptance of these edited citrus varieties. Veronica Ancona and Georgios Vidalakis have also been involved in this part of the project and also coordinate extension and outreach activities in Texas and California respectively, to transfer information and the outcomes of the project to growers and stakeholders.
How does the CRISPR/Cas system work?
The CRISPR/Cas system allows scientists to precisely alter DNA sequences in order to modify gene function, a process called genome editing. This process is performed by causing a break in the DNA and using the cell’s natural DNA repair machinery to introduce changes. As the DNA sequence in a gene codes for a particular protein, changing the DNA sequence causes a change in the protein that is made. Im
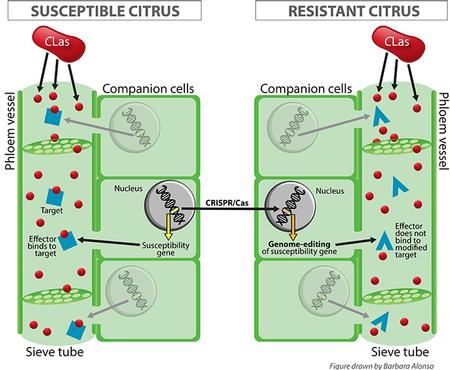 portantly, in the editing process foreign genes are not introduced; rather existing genetic information is rearranged. Since the newly edited variety does not contain any DNA from other organisms, it is not regulated by the USDA as a genetically modified organism (GMO). Genome editing overcomes some problems associated with traditional breeding, such as the long time to complete crosses, slow plant growth, pollen incompatibility, and other constraints particularly severe in citrus.
portantly, in the editing process foreign genes are not introduced; rather existing genetic information is rearranged. Since the newly edited variety does not contain any DNA from other organisms, it is not regulated by the USDA as a genetically modified organism (GMO). Genome editing overcomes some problems associated with traditional breeding, such as the long time to complete crosses, slow plant growth, pollen incompatibility, and other constraints particularly severe in citrus.In this project, the CRISPR/Cas system is being used to modify citrus genes targeted by CLas effectors, attempting to make citrus proteins less susceptible to bacterial effectors, and consequently making citrus more tolerant or resistant to HLB (Figure 1).
Who is working on the project?
This project, funded by the USDA-NIFA Specialty Crop Research Initiative, is a collaborative effort between Wenbo Ma and Georgios Vidalakis from the University of California, Riverside; Gitta Coaker from the University of California, Davis; Nian Wang and Lisa House from the University of Florida; and Veronica Ancona from Texas A&M University-Kingsville. Some of the preliminary studies were supported by the Citrus Research Board.
What are the challenges and opportunities?
Development of resistant or tolerant varieties is considered to be the most sustainable strategy to control plant diseases. However, no naturally occurring resistance against HLB has been found in cultivated citrus varieties. The CRISPR/Cas system is a powerful new tool to generate the much-needed resistance by identifying and modifying specific citrus genes targeted by CLas effectors, so CLas can no longer infect and/or cause damage to citrus. However, the success of this strategy depends on accurate characterization of the molecular interactions between CLas and citrus, so that appropriate genes to be modified in citrus can be identified. For this newly developed resistance to be durable, it is necessary to predict ways in which the bacterium might overcome the resistance during the co-evolutionary “arms race” with plant hosts, and design strategies to prevent this. Finally, although citrus varieties obtained through genome editing are not considered GMOs from a regulatory standpoint, consumers need to understand the CRISPR/Cas technology to help increase their acceptance of edited citrus varieties.
Funding source: This project is funded by the Citrus Research Board, and the USDA-NIFA Specialty Crop Research Initiative.
-
Using tristeza virus to provide citrus with anti-microbial or insecticidal
Research by Drs. Bill Dawson and Kirsten Pelz-Stelinski, University of Florida
Article written by Bill Dawson, Kirsten Pelz-Stelinski, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised June 27, 2025.What is the technique?
A novel way to kill Asian citrus psyllids is to insert into the citrus plant insect-derived or plant-derived peptides. These are short portions of protein that have insecticidal (kill the insect) or antimicrobial (kill CLas) activity. An example of a plant-based insecticidal peptide is lectins, that act against phloem-feeding insects. Lectins affect receptors in the insect gut, causing lesions that prevent the insect from feeding, and so it dies. Antimicrobial peptides affect the internal communities of endosymbiotic (helpful) bacteria in psyllids, reducing psyllid fitness. Antimicrobial peptides also have the potential to kill CLas bacteria directly, if selective antimicrobials can be found, engineered into plants, and delivered to the psyllid.
Using viruses as delivery tools
Antimicrobial peptides could be inserted into citrus via genetic engineering, but this approach requires replanting of existing citrus trees and the process would need to be repeated for each variety of citrus. An alternative method to deliver peptides, is to engineer a mild strain of the citrus tristeza virus (CTV) that, in itself is not damaging to citrus, but it can express insecticidal or antimicrobial peptides. This altered CTV would act as a carrier to inoculate trees and so would not require the trees to be replanted – creating an approach that is much faster than engineering the plant.
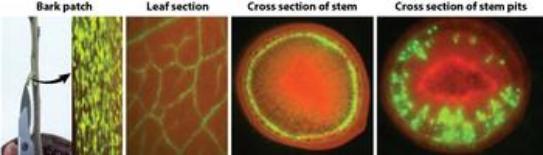
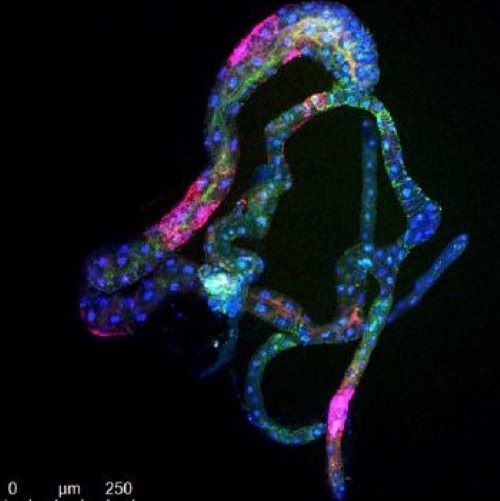
In order to show visually where the virus ends up in the tree, Dawson created a virus that makes a visual green fluorescent protein (GFP). CTV was shown to produce GFP (green color) to very high levels (below) throughout the tree. Recently, Dawson and his collaborators have used the vector to produce a wide range of antimicrobial peptides to control HLB.
Who is working on the Project?
Bill Dawson at the University of Florida, has pioneered research on development of CTV vectors to transport foreign genes within plants. Through his laboratories’ research, CTV vectors have been developed to express foreign genes throughout citrus trees, acting like ‘vehicles’ to deliver antimicrobials into the tree. Dr. Kirsten Pelz-Stelinski, also of the University of Florida is collaborating in learning how the insecticidal peptides affect psyllids.
What are the challenges and opportunities?
Citrus budwood, containing a mild strain of CTV that makes insecticidal or antibacterial peptides specific for psyllids, could be grafted onto nursery trees. Or, the CTV budwood could be graft-inoculated onto existing trees, negating the need for tree removal and replanting, and would not require developing new technologies for different varieties. Because making the peptide is not a permanent ability of the virus, it eliminates the possibility of the trait escaping in pollen. The major limiting factors in developing this technology are finding effective antimicrobial and insecticidal peptides to incorporate into the CTV and obtaining approval for their use from the FDA and EPA. To this end, on May 10, 2018 USDA APHIS released a draft Environmental Impact Statement for a 45 d period for public comment (https://www.regulations.gov/docket?D=APHIS-2017-0018). This document describes the environmental impacts that might result from the environmental release throughout Florida of genetically engineered Citrus tristeza virus. They also included an analysis of the plant pest risks associated with using this engineered virus as a biological control agent to help manage HLB.
Citation: Dawson, W.O., Bar-Joseph, M., Garnsey, and Moreno, P. 2015. Citrus Tristeza Virus: Making an Ally from an Enemy. Annual Review of Phytopathology 53: 137-155. http://www.annualreviews.org/doi/full/10.1146/annurev-phyto-080614-120012
Funding source: This project is funded by USDA-NIFA.
-
Founder lines used to improve HLB tolerance
Research by Dr. James Thomson, USDA-Agricultural Research Service, Albany, CA
Article written by James Thomson, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised June 27, 2025.What is the technique?
“Founder Lines” can be used to genetically engineer citrus plants with desirable traits, like tolerance to huanglongbing (HLB). But, what are Founder Lines? Founder lines are plants that have a DNA sequence, or piece of genetic material, that is used as a docking site for the insertion of one or more genes for desired traits at specific locations in the genome. Having a docking site ensures that insertions do not interrupt desirable genes, that high and consistent activity of the inserted sequence is achieved, which is more likely if only a single copy of the gene is introduced. The approach also leads to removal of marker genes for antibiotic resistance, that are needed to identify engineered tissues, and to reduce the time and cost of creating engineered varieties.
How is genetic engineering involved?
The Founder line approach uses primarily citrus genes to genetically engineer citrus varieties in a very precise way. The first step is creating a citrus line with target sites inserted in its genome, and the first founder line utilized the Carrizo variety. DNA is introduced into young stem slices and then, using appropriate media, individual cells in the stem are “coaxed” to form plantlets (see below).
DNA can be introduced into cells in the slice in several ways. First it can be done via an electrical current, which pokes holes in the cell wall, allowing DNA to enter and insert permanently into the genome. Secondly, DNA can be introduced via a natural soil-born organism, Agrobacterium tumefaciens*, which can inject into plant cells DNA that inserts into the genome.
Once the target sites are identified and validated, they can be used for future docking efforts. This is because the docking site is recognized by special enzymes, called recombinases, which specifically identify the DNA sequence (recognition site) within the docking site and insert new genetic information at that location. The recombinase is so precise that the new information can be int
 roduced without gaining or losing even one single chemical unit of DNA (Figure 1).
roduced without gaining or losing even one single chemical unit of DNA (Figure 1).The next part of the process is introducing recognition sites that are used by the recombinase to insert the new genetic material, such as HLB resistance, into the site and to remove the marker gene(s).
The same strategy can be used to “stack” additional traits into the same location, using the target sites and the recombinase. For example, an existing trait for HLB resistance can be stacked with another trait for resistance to the fungal pathogen Phytophthora.
Who is working on the Project?
This approach is being developed by James Thomson, a research scientist at the USDA-ARS in Albany CA. To date, through collaborative efforts with other scientists around the country, such as Ed Stover at USDA-ARS Ft. Pierce FL, Eliezer Louzada at Texas A&M, Gitta Coaker at UC Davis and William Belknap at USDA-ARS in Albany, 19 potential resistance genes have been identified and are being introduced into citrus as single genes and in some cases “stacked” as multigene cassettes. Once completed, the engineered plants will be given to collaborators for disease resistance evaluation.
What are the challenges and opportunities?
Although initial introductions into citrus of DNA with the target sites are complete and many different lines have been micro-grafted onto plants in the greenhouse, these must be characterized to select “Founder Lines” with the desired characteristic of HLB disease resistance. The team is also focusing on improving transformation/regeneration efficiency and rates of recombinase-mediated gene insertion. In other words, they are trying to get more plants with DNA where they want it with less effort.

One important benefit of the Founder Line technique is the fact that the time and effort for federal regulatory oversight could be reduced because introduced traits will always be inserted into the same location in the genome.
Funding source: Founder Line development and RMCE genome targeting research development is supported by the USDA-ARS and the Citrus Research Board.
-
Disease resistance in citrus with addition of plant defense genes
Research by Drs. Manjul Dutt and Jude Grosser, University of Florida
Article written by Manjul Dutt, Jude Grosser, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised June 27, 2025.What is the technique?
Many plants defend themselves from pathogens (and insect feeding) by producing proteins that enhance their resistance to these organisms. This is called systemic acquired resistance (SAR). Specific defense genes code for pathogenesis related (PR) proteins that protect plants from microorganisms and are induced by a plant hormone called salicylic acid. Genes from resistant or tolerant plants can be inserted into less tolerant ones, thereby improving disease resistance traits in those varieties that are highly susceptible to disease. This use of genetic engineering is a relatively fast method of improving existing cultivated plants by enhancing their resistance to newly introduced pathogens.
How is genetic engineering involved?
The model plant, Arabidopsis, was used in this research. A gene called Non-expressor of Pathogen Related genes 1 (NPR1) has been identified in this plant. Arabidopsis mutants that lack expression of the NPR1 gene do not respond to pathogens by producing protective proteins, while engineered plants over-expressing NPR1 genes have enhanced resistance to several pathogens, ranging from fungal to bacterial. Researchers have recently taken this approach to protect citrus from huanglongbing disease (HLB). They developed transgenic trees that overexpress an NPR1 gene in the phloem tissues (where the HLB pathogen occurs) of both ‘Hamlin’ and “Valencia’ sweet oranges. These varieties are the most predominant sweet oranges grown in Florida and Brazil for juicing.

Transgenic citrus trees were developed by inserting a phloem-specific Arabidopsis gene promoter (gene region that drives production of the NPR1 protein). This ensures that overexpression of these NPR1 defense genes happens in that tissue. The idea was to engineer normally susceptible plants to be resistant to HLB by boosting their natural ‘immune’ defense to this disease. The transformed plants were propagated and grafted onto a common rootstock. Plants transformed to express resistance to HLB were evaluated by exposing them in the laboratory to free-flying Asian citrus psyllids that were carrying the pathogen. Also, transgenic plants (and non-transformed controls) were planted in citrus groves with a high (>90%) incidence of disease. The researchers found that by overexpressing an NPR1 gene they created citrus trees that were more resistant to HLB than controls. A few of the tested transgenic lines remained free of disease for up to 36 months after exposure in the field investigation. In comparison, control plants became infected after only 6 months in the field.
Who is working on the Project?
Drs. Manjul Dutt and Jude Grosser conceived, designed, and have led this research. They lead laboratories at the University of Florida’s Citrus Research and Education Center in Lake Alfred, Florida.
What are the challenges and opportunities?
This research points to one possible important tool for combating HLB directly with transgenic trees that show increased natural immunity to the disease. Transgenic plants are in many ways similar to plants released through traditional breeding methods. However, consumer education regarding their safety continues to be needed. Research is also underway to examine if transforming the rootstock of commercial citrus trees alone without manipulating the scion (fruiting portion of the tree) might have the same effects. The idea is to boost the immunity of the entire tree by genetically engineering only the root portion of the commercial tree. In this manner, the scion, which is grafted on later and grows above ground to produce fruit, would not express any of the engineered genes and therefore harvested fruit would not be considered engineered.
Source: Dutt M, Barthe G, Irey M, Grosser J (2015) Transgenic Citrus Expressing an Arabidopsis NPR1 Gene Exhibit Enhanced Resistance against Huanglongbing (HLB; Citrus Greening). PLoS ONE 10(9): e0137134. doi:10.1371/journal.pone.0137134
Funding source: This project is funded by the Citrus Research and Development Foundation.
-
Using peptides as a preventive approach to target the psyllid and the pathogen
Research by Dr. Robert Shatters, USDA Horticultural Research Laboratory, Fort Pierce, FL
Article written by Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10, 2025.Peptides are short chains that come from naturally occurring proteins. Most organisms produce a special group of peptides that act as a first line of defense against bacteria, interfering with their ability to transmit disease. USDA-ARS researcher, Dr. Robert Shatters, along with others, is looking at the possible use of antimicrobial peptides to stop the spread of the CLas bacterium that causes huanglongbing (HLB) disease of citrus, in concert with peptides that affect the Asian citrus psyllid’s ability to acquire and transmit the pathogenic bacterium. The quickest approach to use these peptides would be in topical applications to the leaves of citrus trees, while a long term approach would be to engineer citrus with the genes to produce the peptides.
What is the technique?
Dr. Shatters and colleagues are pursuing three different application strategies to deliver a combination of peptides with the combined effect of killing the psyllid, or blocking the psyllid from acquiring and transmitting the bacterium, and/or directly killing the bacteri
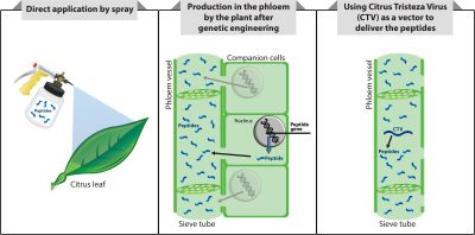 um. The first peptide deliver strategy would be a spray application of peptides directly on the plant. This approach would be the quickest solution for growers; however, it would require repeated applications. A second strategy involves engineering citrus to produce the peptides directly in the phloem, the part of the plant where the bacterium lives. This approach avoids the necessity of having to repeatedly applying the peptides. However, it would work only on new plantings of genetically engineered citrus trees, not on existing trees and the regulatory pathway to permit its commercial use would be longer than direct applications. A third approach , conducted collaboratively with Dr. Bill Dawson, at the University of Florida, involves inserting genes for the peptides into Citrus tristeza virus (CTV). The virus would be transferred to the citrus tree by grafting CTV-infected tissue onto the tree. The engineered CTV would live in the phloem and produce peptides that would circulate through the phloem. This strategy will require only a single application. It could be used on existing plantings and would require a longer regulatory process than the sprayable peptides.
um. The first peptide deliver strategy would be a spray application of peptides directly on the plant. This approach would be the quickest solution for growers; however, it would require repeated applications. A second strategy involves engineering citrus to produce the peptides directly in the phloem, the part of the plant where the bacterium lives. This approach avoids the necessity of having to repeatedly applying the peptides. However, it would work only on new plantings of genetically engineered citrus trees, not on existing trees and the regulatory pathway to permit its commercial use would be longer than direct applications. A third approach , conducted collaboratively with Dr. Bill Dawson, at the University of Florida, involves inserting genes for the peptides into Citrus tristeza virus (CTV). The virus would be transferred to the citrus tree by grafting CTV-infected tissue onto the tree. The engineered CTV would live in the phloem and produce peptides that would circulate through the phloem. This strategy will require only a single application. It could be used on existing plantings and would require a longer regulatory process than the sprayable peptides.Who is working on the project?
Dr. Robert Shatters, a Research Molecular Biologist at the USDA Horticultural Research Laboratory in Ft. Pierce FL, and his laboratory have demonstrated that by combining antimicrobial peptides with acquisition/transmission blocking peptides, up to 90% of the psyllids were killed in lab experiments and pathogenic bacteria were completely blocked from being acquired by the psyllids that survived. This group is now moving this work to greenhouse and research field to determine how well these peptides function under whole-plant and field conditions. This work is being conducted collaboratively with other scientists. Dr. Michelle Cilia, USDA-ARS in Ithaca NY, is identifying the specific protein interactions involved with the gut binding peptides; Dr. Ed Stover, USDA-ARS, Fort Pierce FL, is working to produce transgenic citrus expressing these peptides; and Dr. Bill Dawson, University of Florida, is investigating use of the CTV virus to produce these peptides within infected citrus.
What are the challenges and opportunities?
Using peptides to reduce disease is not a new technique, as they are currently being used in human health to attempt to combat damage from cardiovascular disease.
Because citrus is constantly being re-infected with the CLas bacterium, this approach will most likely serve as a preventative. However, in California, where psyllid and tree infection rates are very low, it is possible this strategy could be a cure. Experimentally, bacterial populations were reduced by peptides in citrus leaves by >99% after five days (based on detectable bacterial DNA). Thus, over time, treatment with peptides could result in a cure, if peptides were shown to reach throughout the plant, including the roots.
In order for these approaches to be effective in reducing HLB in citrus, a number of parts to this research puzzle need to fall into place. First, the peptides have to be effective against the bacterium. Second, the delivery of the peptides into the psyllid needs to be effective and economically viable. Third, to be useful for growers, the regulatory pathway must be established that will permit these tools to be deployed in the field. It is encouraging that commercial peptide-based topical sprays were developed and used by growers in the 1990’s to induce plant defenses. And, since then, more economical methods of producing peptides have been achieved. If these three hurdles can be overcome, peptides may become a useful tool to combat HLB.
-
Growing citrus under enclosures
Research by Dr. Rhuanito Soranz Ferrarezi, University of Florida
Article written by Rhuanito Soranz Ferrarezi, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10, 2025.What is the technique?
Citrus production under protected environments reduces huanglongbing (HLB) disease incidence and damage by excluding the Asian citrus psyllid (ACP) vector from access to citrus trees by surrounding them with a screen barrier. In addition to protecting citrus from the disease, it greatly reduces insecticidal sprays applied to control psyllids. This reduces costs and selection for insecticide resistance in the psyllid.
How does it improve HLB management?

Completely enclosed screenhouses physically exclude the ACP and thus prevent inoculation of the plants with the CLas bacteria that causes HLB. A screened enclosure in Florida fully protected young grapefruit (Citrus × paradisi) trees from eggs, nymphs and adult ACP and no trees tested positive for CLas inside the enclosure after two years of monitoring. In contrast 100% of surveyed trees in the nearby open-air plots tested positive for CLas during the same period. Thus, the use of screenhouses offered a substantial level of protection against the establishment of HLB, compared to management programs founded solely upon protecting the citrus from ACP using insecticidal sprays.
Who is working on the Project?
The citrus under protective screen system was developed at the University of Florida/Institute of Food and Agricultural Sciences (UF/IFAS) Indian River Research and Education Center in Fort Pierce, FL and has been tested at the UF/IFAS Citrus Research and Education Center in Lake Alfred, FL. The economics of citrus under protective screens (CUPS) is being determined. To date, there are 50 acres of commercial CUPS with three growers in Florida, and at least 150 more acres are planned (Eduardo Pines and Steven Callaham, personal communication).
Drs. Rhuanito Ferrarezi, Arnold Schumann, Ariel Singerman, Jawwad Qureshi, Philippe Rolshausen (UC Davis), Andrew Krajewski (International Citrus Technologies, Australia), Elizabeth Grafton-Cardwell, Stephen Futch, Chris Oswalt and Garima Kakkar are currently part of this research.
Dr. Ferrarezi is with the University of Florida Horticultural Sciences Department He is a professor of citrus horticulture and is located at the Indian River Research and Education Center in Fort Pierce, FL. All other researchers with the exceptions of Grafton-Cardwell (UC Riverside), Krajewski (International Citrus Technologies, Australia), and Rolshausen (UC Davis) are at the University of Florida.
What are the challenges and opportunities?
Researchers are still answering many questions about the technology, such as the most suitable citrus varieties, the installation and maintenance costs, the economics and level of fruit production, the level of labor needed, pest and disease control, resistance to extreme weather conditions such as frost, tropical storms and hurricanes, irrigation and fertilization, canopy management and other technical aspects. The Florida CUPS group recently received a USDA-NIFA-CDRE grant to address a number of these topics.
Ferrarezi RS, Wright AL, Boman BJ, Schumann AW, Gmitter FG, Grosser JW. 2017. Protected fresh grapefruit cultivation systems: Antipsyllid screen effects on environmental variables inside enclosures. HortTechnology 27(5): 675-681.
http://dx.doi.org/10.21273/HORTTECH03790-17Ferrarezi RS, Wright AL, Boman BJ, Schumann AW, Gmitter FG, Grosser JW. 2017. Protected fresh grapefruit cultivation systems: Antipsyllid screen effects on plant growth and leaf transpiration, vapor pressure de?cit, and nutrition.
HortTechnology 27(5): 666-674. http://dx.doi.org/10.21273/HORTTECH03789-17
Funding source: This project is funded by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2018-70016-27387. -
HLB control: capitalizing on resistance in Poncirus trifoliata to Asian citrus psyllid
Research by Dr. David Hall, USDA-ARS, Fort Pierce, FL
Article written by David Hall, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 9, 2025.What is the technique?
All Citrus species are susceptible to colonization by large numbers of the Asian citrus psyllid (ACP). In contrast, Poncirus trifoliata is a genotype with strong resistance to ACP infestations. Poncirus and Citrus are closely related and readily hybridized. Research is therefore being conducted to identify traits responsible for ACP resistance in Poncirus and to determine if these traits can be passed to hybrids with Citrus species or utilized in other ways to reduce ACP infestation and HLB in citrus.

How does it improve HLB management?
Current recommendations to growers confronted with HLB are to plant disease-free nursery stock, routinely identify and remove infected trees to reduce inoculum loads and aggressively manage populations of the psyllid. Insecticidal control is the key tactic used to manage the psyllid, but host plant resistance holds some promises or clues in the search for alternative tactics. Field, laboratory and greenhouse investigations have confirmed that ACP infestations are greatly reduced on Poncirus. One trait involved has already been identified: Poncirus leaves emit volatiles that discourage oviposition. The specific volatiles involved are being investigated. There may be other chemical traits that work in concert with these volatiles to reduce ACP colonization of Poncirus. If the chemicals that deter ACP infestations can be identified, they might be applied directly to reduce infestations in citrus. Further, the genes responsible for producing these chemicals in Poncirus might be transferred to citrus through conventional breeding.
Who is working on the project?
David Hall, Steve Lapointe and Ed Stover have led research efforts to develop management tactics for ACP based on resistant traits associated with Poncirus. They are with USDA’s Agricultural Research Service, U.S. Horticultural Research Laboratory located in Fort Pierce, FL.
What are the challenges and opportunities?
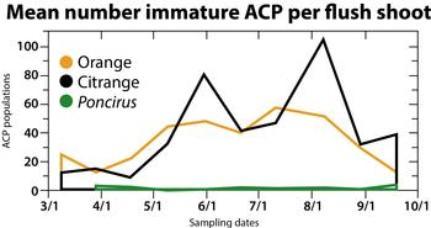 This research is being pursued in search of new tactics for managing ACP in citrus, to reduce insecticide use and assist in HLB control. Although closely related to Citrus, Poncirus differs in that it is deciduous during the winter and produces inedible fruit. The resistance in Poncirus to ACP infestations is striking. For example, in a recent field study averages of 2 and 40 immature ACP (eggs and nymphs combined) per flush shoot were observed during the summer in 5-year-old Poncirus and sweet orange trees, respectively (see chart below). A similar level of plant resistance to ACP in citrus would be expected to reduce area-wide populations of the psyllid and consequently the incidence of HLB in citrus. A very limited number of citrange cultivars (hybrids between Poncirus and sweet orange) have thus far been evaluated for ACP resistance, and these did not exhibit resistance. The search needs to be expanded to a larger number of hybrids. In the meantime, we may be successful in identifying volatiles and other chemicals associated with Poncirus that suppress oviposition. These may have value as foliar sprays for reducing ACP infestations.
This research is being pursued in search of new tactics for managing ACP in citrus, to reduce insecticide use and assist in HLB control. Although closely related to Citrus, Poncirus differs in that it is deciduous during the winter and produces inedible fruit. The resistance in Poncirus to ACP infestations is striking. For example, in a recent field study averages of 2 and 40 immature ACP (eggs and nymphs combined) per flush shoot were observed during the summer in 5-year-old Poncirus and sweet orange trees, respectively (see chart below). A similar level of plant resistance to ACP in citrus would be expected to reduce area-wide populations of the psyllid and consequently the incidence of HLB in citrus. A very limited number of citrange cultivars (hybrids between Poncirus and sweet orange) have thus far been evaluated for ACP resistance, and these did not exhibit resistance. The search needs to be expanded to a larger number of hybrids. In the meantime, we may be successful in identifying volatiles and other chemicals associated with Poncirus that suppress oviposition. These may have value as foliar sprays for reducing ACP infestations.Funding source: This project is funded by the Citrus Research & Development Foundation.