The Juvenile Tissue Citrus Transformation Facility (JTCTF) has undergone a transition to Educational Business Activity unit in accordance with request made by UF Administration. This transition completely overhauls the operation of the facility and brings into force new set of rules in regard to ordering, pricing, and payments to the JTCTF for the services it provides.
Mission
The Juvenile Tissue Citrus Transformation Facility was established to expedite integration of recombinant DNA technologies and genetic engineering into basic research from other related disciplines, including plant pathology, biochemistry, food science/processing, post-harvest physiology, horticulture, and entomology by providing a service that allows researchers to have their genes of interest transferred and tested in the appropriate citrus cultivars. A functional core citrus transformation laboratory benefits nearly all phases of the Florida citrus industry and plays a major role in the long-term improvement of citrus.
Purpose
The purpose of the JTCTF is to produce genetically modified Citrus plants. The facility is equipped to employ two methods of genetic transformation: Agrobacterium-mediated transformation and biolistic particle bombardment. At this time we are producing transgenic plants only through Agrobacterium-mediated transformation. This method utilizes naturally occurring pathogenic bacterium called Agrobacterium tumefaciens that has the ability to insert the fragment of DNA into the genome of host plant. These bacteria have been manipulated and rendered non-pathogenic although they kept their ability to transfer foreign DNA into host’s genome. The DNA fragment of interest to our client is introduced into Agrobacterium, and such newly created strain is used for treatment of plant tissues that will result in production of plantlets that have their genomes altered by the presence of foreign DNA. Additional tests are performed to confirm that genetic modification took place.
-
Accomplishments
Core Citrus Transformation Facility (CCTF) exists for more than 16 years. Since becoming fully functional 14 years ago, CCTF has succeeded in fulfilling the role of becoming the production site for transgenic Citrus plants. Clients from many states within the US and, from different research organizations and foundations have come to us and requested plants of Citrus cultivars of choice with the desired gene inserted into their genome. Despite detrimental effect of HLB on transformation success rate and exploratory nature of many recent orders, CCTF is still providing transgenic material at satisfactory level. At the moment, capacity of CCTF is to produce about 250 seedlings per year although the final number depends on the cultivars that are ordered/nature of the orders placed.
Most of the plants produced in the CCTF within the last few years were transgenic ‘Duncan’ grapefruit, followed by Carrizo citrange, sweet orange 'Valencia' and ‘Pineapple’ cultivars, Mexican lime, and Swingle citrumelo.
Based on the un-interrupted operation, previous accomplishments, and productivity CCTF has become integral part of many projects that have a goal of production of tolerant/resistant trees to HLB, canker, and CTV.
CCTF is recognized outside of the US as well, with many scientists quoting the publications that were published based on experiments done on transgenic plants made in our facility.
As our major accomplishments I would list the following:
- we were the first lab to produce cis/intragenic Citrus plants;
- we were the first place to produce transgenic Citrus plants with chemically inducible genes;
- we were the first place where transgenic Citrus plants with CRISPR-edited genome were produced.
-
Citrus Transformation Phases

Sterilized seeds are allowed to germinate in darkness for 4-5 weeks.
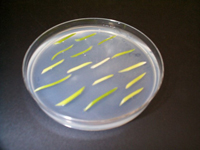
Stems of seedlings grown in vitro are cut into 1/2 inch explants, incubated with Agrobacteria and placed on shoot regeneration medium.

Shoots appear in explants (4-5 weeks after treatment with bacteria)

Shoots are collected from explants and allowed to grow for a week.

Tested for reporter genes.

Shoots grafted on rootstocks in vitro.

Two weeks old, soil adapted seedlings

Four months seedlings
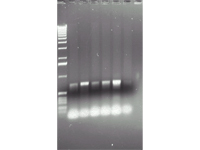
Gel with the products of PCR reaction proving that the gene of interest is present within the tissue of transgenic plants
-
Equipment
CCTF is located on the campus of CREC. The laboratory has standard equipment including:
- 3 laminar flow hoods
- Autoclave
- Sterilizing furnace
- Incubator
- Thermo-controlled shaker
- Zeiss microscopes including inverted stereo microscope and binocular microscope equipped with digital camera which uses SPOT software for fluorescence analysis
Plants and explants are kept in 2 new Percival reach-in growth chambers, and in a greenhouse certified for transgenic material.
-
Podcast
Here you will find regularly-updated podcast presentations. You will also be able to read a transcription of the podcast, and view the original articles and relevant materials.
-
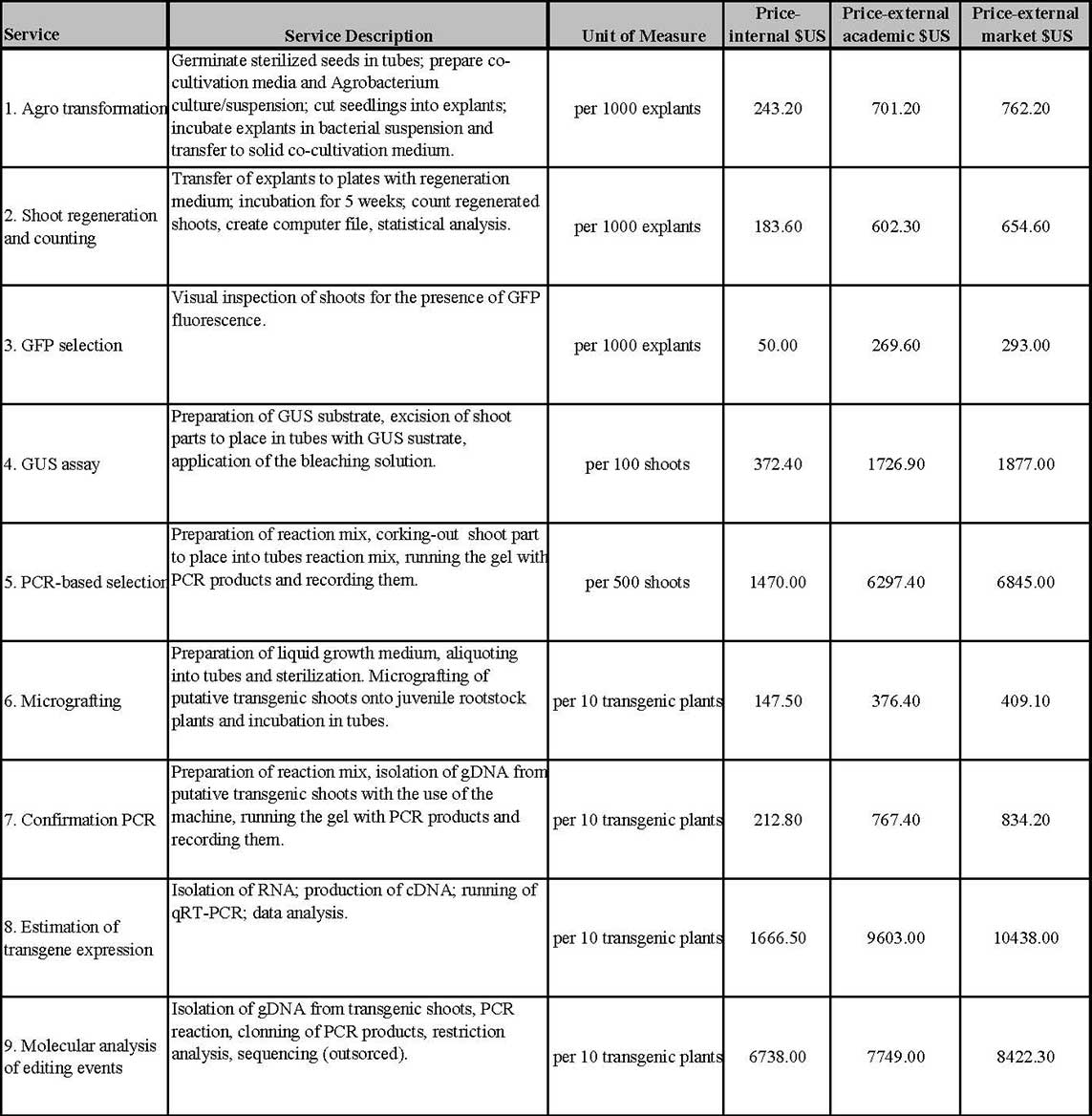
Price Lists
The transition of JTCTF into the EBA unit has changed the way we charge for our services. Please inspect the pricelist below for additional information. The prices marked “internal academic” refer to orders coming from the University of Florida (UF). Those prices marked “external academic” refer to orders coming from other academic institutions, whereas the prices in the column “external market” are for business entities. When hiring the JTCTF, clients will sign the contract that will be supplied by the office of Business Management at UF. Starting with January 1st 2021, JTCTF will bill the clients on a monthly basis. Before we start working on the order, we will provide an approximate total price for the sought service(s). Monthly billing has a purpose of keeping the facility funded throughout the whole year. It also means that clients are assuming the responsibility for the success of the designed experiments. In other words, JTCTF will require payments for the work performed in a certain time frame regardless of the outcome of those efforts. Because of the exploratory nature of these experiments, it will take three months to estimate possible detrimental effect of introduced gene (sensu lato) on regeneration of shoots from explants co-incubated with Agrobacterium and JTCTF will charge clients for the work it performed in that initial period. If the goal of the introduction of transgene into citrus plants is to change phenotypic features that can be recorded by visual inspection, then JTCTF will communicate the success in realized project to clients as the experiments move ahead. Clients will have the chance to stop the work done by JTCTF if there is a lack of progress. In the case where the function of the transgene in transgenic plants needs to be confirmed by additional tests, the responsibility of performing such tests lays on the client except if they wish to hire JTCTF to perform one of the services listed in the Pricelist. It will take 9-12 months, depending on the cultivar, to produce plants that could be tested for the functionality of introduced transgene and JTCTF will require payments for the work done towards production of transgenic plants regardless of the outcome of those tests.
-
Links
- USDA's Agricultural Biotechnology
- Oklahoma Plant Transformation Facility, Nobel Res Ctr
- UCBiotech - University of California
- USDA Biotechnology Permit Information
- Plant Transformation Core Facility, Univ. of Missouri
- Plant Tissue Culture and Transformation Facility, Cornell
- Plant Biotechnology Core Facility, Univ. of Connecticut
- Plant Biotechnology Facility, University of Wisconsin
-
Literature 2021-2019
2021
Sinn JP, Held JB, Vosburg C, Klee SM, Orbovic V, Taylor EL, Gottwald TR, Stover E, Moore GA, McNellis TW. Flowering Locus T chimeric protein induces floral precocity in edible citrus. Plant Biotechnol J. 2021 Feb;19(2):215-217. https://doi.org/10.1111/pbi.13463. Epub 2020 Sep 16.
2020
Conti G, Gardella V, Vandecaveye MA, Gomez CA, Joris G, Hauteville C, Burdyn L, Almasia NI, Nahirñak V, Vazquez-Rovere C, Gochez AM, Furman N, Lezcano CC, Kobayashi K, García ML, Canteros BI, Hopp HE, Reyes CA. Transgenic Citrange troyer rootstocks overexpressing antimicrobial potato Snakin-1 show reduced citrus canker disease symptoms. J Biotechnol. 2020 Dec 20;324:99-102. https://doi.org/10.1016/j.jbiotec.2020.09.010. Epub 2020 Sep 28.
Corte, L.E., B.M.J. Mendes, F.A.A. Mourao Filho, J.W. Grosser and M. Dutt (2020). Functional characterization of full-length and 5′ deletion fragments of Citrus sinensis-derived constitutive promoters in Nicotiana benthamiana. In Vitro Cellular and Developmental Biology – Plant https://doi.org/10.1007/s11627-019-10044-0
Dutt, M., Mou, Z., Zhang, X. et al. Efficient CRISPR/Cas9 genome editing with Citrus embryogenic cell cultures. BMC Biotechnol 20, 58 (2020). https://doi.org/10.1186/s12896-020-00652-9
Dasgupta K, Hotton S, Belknap W, Syed Y, Dardick C, Thilmony R, Thomson JG. Isolation of novel citrus and plum fruit promoters and their functional characterization for fruit biotechnology. BMC Biotechnol. 2020 Aug 20;20(1):43. https://doi.org/10.1186/s12896-020-00635-w
Jardak, R., Boubakri, H., Zemni, H. et al. Establishment of an in vitro regeneration system and genetic transformation of the Tunisian 'Maltese half-blood' (Citrus sinensis): an agro-economically important variety. 3 Biotech 10, 99 (2020). https://doi.org/10.1007/s13205-020-2097-6
Mahmoud, L.M., J.W. Grosser and M. Dutt (2020). Silver compounds regulate leaf drop and improve in vitro regeneration from mature tissues of Australian finger lime (Citrus australasica). Plant Cell Tissue and Organ Culture. https://doi.org/10.1007/s11240-020-01803-8
Pereira W, Takita M, Melotto M, de Souza A. Citrus reticulata CrRAP2.2 Transcriptional Factor Shares Similar Functions to the Arabidopsis Homolog and Increases Resistance to Xylella fastidiosa. Mol Plant Microbe Interact. 2020 Mar;33(3):519-527. https://www.doi.org/10.1094/MPMI-10-19-0298-R. Epub 2020 Jan 23. PMID: 31973654.
Poles L, Licciardello C, Distefano G, Nicolosi E, Gentile A, La Malfa S. Recent Advances of In Vitro Culture for the Application of New Breeding Techniques in Citrus. Plants (Basel). 2020 Jul 24;9(8):938. https://doi.org/10.3390/plants9080938.
Qiu, W., J. Soares, Z. Pang, Y. Huang, Z. Sun, N. Wang, J.W. Grosser and M. Dutt (2020). Potential Mechanisms of AtNPR1 Mediated Resistance against Huanglongbing (HLB) in Citrus. International journal of molecular sciences 2020, 21, 2009. https://doi.org/10.3390/ijms21062009
Romero-Romero, J.L., Inostroza-Blancheteau, C., Reyes-Díaz, M. et al. Increased Drought and Salinity Tolerance in Citrus aurantifolia (Mexican Lemon) Plants Overexpressing Arabidopsis CBF3 Gene. J Soil Sci Plant Nutr 20, 244–252 (2020). https://doi.org/10.1007/s42729-019-00130-y
Soares, J.M., Weber, K.C., Qiu, W. et al. The vascular targeted citrus FLOWERING LOCUS T3 gene promotes non-inductive early flowering in transgenic Carrizo rootstocks and grafted juvenile scions. Sci Rep 10, 21404 (2020). https://doi.org/10.1038/s41598-020-78417-9
Ying X, Redfern B, Gmitter FG Jr, Deng Z. Heterologous Expression of the Constitutive Disease Resistance 2 and 8 Genes from Poncirus trifoliata Restored the Hypersensitive Response and Resistance of Arabidopsis cdr1 Mutant to Bacterial Pathogen Pseudomonas syringae. Plants (Basel). 2020 Jun 30;9(7):821. https://doi.org/10.3390/plants9070821.
Zhang, F., Rossignol, P., Huang, T., & Irish, V. (2020). Reprogramming of Stem Cell Activity to Convert Thorns into Branches. Current Biology, 30, 2951-2961.e5.
Zhang, XH., Pizzo, N., Abutineh, M. et al. Molecular and cellular analysis of orange plants infected with Huanglongbing (citrus greening disease). Plant Growth Regul 92, 333–343 (2020). https://doi.org/10.1007/s10725-020-00642-z
2019
Ćalović, M., Chen, C., Yu, Q., Orbović, V., Gmitter, F. G., & Grosser, J. W. (2019). New Somatic Hybrid Mandarin Tetraploid Generated by Optimized Protoplast Fusion and Confirmed by Molecular Marker Analysis and Flow Cytometry, Journal of the American Society for Horticultural Science J. Amer. Soc. Hort. Sci., 144(3), 151-163. Retrieved Mar 24, 2021, from https://journals.ashs.org/jashs/view/journals/jashs/144/3/article-p151.xml
Goulin, E.H., dos Santos, P.J.C., Dalio, R.D. et al. In vitro symptom induction of Colletotrichum abscissum infection in detached sweet orange flowers. J Plant Pathol 101, 695–699 (2019). https://doi.org/10.1007/s42161-018-00220-3
Jia H, Orbović V, Wang N. CRISPR-LbCas12a-mediated modification of citrus. Plant Biotechnol J. 2019 Oct;17(10):1928-1937. https://doi.org/10.1111/pbi.13109. Epub 2019 Apr 10.
Peng, A., Zou, X., Xu, L. et al. Improved protocol for the transformation of adult Citrus sinensis Osbeck ‘Tarocco’ blood orange tissues. In Vitro Cell.Dev.Biol.-Plant 55, 659–667 (2019). https://doi.org/10.1007/s11627-019-10011-9
Soriano, Leonardo, Tavano, Eveline Carla da Rocha, Correa, Marcelo Favaretto, Harakava, Ricardo, Mendes, Beatriz Madalena Januzzi, & Mourão Filho, Francisco de Assis Alves. (2019). In vitro organogenesis and genetic transformation of mandarin cultivars. Revista Brasileira de Fruticultura, 41(2), e-116. Epub April 25, 2019.https://doi.org/10.1590/0100-29452019116
Tavano, E.C., Erpen, L., Aluisi, B., Harakava, R., Lopes, J., Vieira, M.C., Piedade, S.M., Mendes, B., & Filho, F.A. (2019). Sweet orange genetic transformation with the attacin A gene under the control of phloem-specific promoters and inoculation with Candidatus Liberibacter asiaticus. The Journal of Horticultural Science and Biotechnology, 94, 210 - 219. https://doi.org/10.1080/14620316.2018.1493361
Wang, L., Chen, S., Peng, A. et al. CRISPR/Cas9-mediated editing of CsWRKY22 reduces susceptibility to Xanthomonas citri subsp. citri in Wanjincheng orange (Citrus sinensis (L.) Osbeck). Plant Biotechnol Rep 13, 501–510 (2019). https://doi.org/10.1007/s11816-019-00556-x
Wu, Hao; Acanda, Yosvanis; Canton, Michel; Zale, Janice. 2019. "Efficient Biolistic Transformation of Immature Citrus Rootstocks Using Phosphomannose-isomerase Selection" Plants 8, no. 10: 390. https://doi.org/10.3390/plants8100390
Zhu, C., Zheng, X., Huang, Y., Ye, J., Chen, P., Zhang, C., Zhao, F., Xie, Z., Zhang, S., Wang, N., Li, H., Wang, L., Tang, X., Chai, L., Xu, Q. and Deng, X. (2019), Genome sequencing and CRISPR/Cas9 gene editing of an early flowering Mini‐Citrus (Fortunella hindsii). Plant Biotechnol J, 17: 2199-2210. https://doi.org/10.1111/pbi.13132
-
Literature 2018-2015
2018
Chen, L., Li, W., Katin-Grazzini, L. et al. A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Hortic Res 5, 13 (2018). https://doi.org/10.1038/s41438-018-0023-4
Dutt M., F.T. Zambon, L. Erpen, L. Soriano, J.W. Grosser (2018). Embryo-specific expression of a visual reporter gene as a selection system for citrus transformation. PLOS ONE 13 (1):e0190413..pone.0190413. https://doi.org/10.1371/journal.pone.0190413
Dutt, M., L. Erpen, and J.W. Grosser, 2018. Genetic transformation of the ‘W Murcott’ tangor: comparison between different techniques. Scientia Horticulturae, 242:90-94. https://doi.org/10.1016/j.scienta.2018.07.026
Erpen L., H.S. Devi, J.W. Grosser and M. Dutt (2018). Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell, Tissue and Organ Culture 132 (1):1-25. https://doi.org/10.1007/s11240-017-1320-6
Erpen, L., E.C.R. Tavano, R. Harakava, M. Dutt, J.W. Grosser, S.M.S. Piedade, B.M.J. Mendes and F.A.A. Mourao Filho (2018) Isolation, characterization, and evaluation of three Citrus sinensis-derived constitutive gene promoters. Plant Cell Reports 37(8): 1113–1125. https://doi.org/10.1007/s00299-018-2298-1
Guerra-Lupián MA, Ruiz-Medrano R, Ramírez-Pool JA, Ramírez-Ortega FA, López-Buenfil JA, Loeza-Kuk E, Morales-Galván O, Chavarin-Palacio C, Hinojosa-Moya J, Xoconostle-Cázares B. Localized expression of antimicrobial proteins mitigates huanglongbing symptoms in Mexican lime. J Biotechnol. 2018 Nov 10;285:74-83. https://doi.org/10.1016/j.jbiotec.2018.08.012. Epub 2018 Sep 5.
Hijaz, F., Y., Nehela, S. E., Jones, M. Dutt, J. W., Grosser, J. A., Manthey and N. Killiny. 2018. Metabolically engineered anthocyanin-producing lime provides additional nutritional value and antioxidant potential to juice. Plant Biotechnology Reports, 12(5):329-346. https://doi.org/10.1007/s11816-018-0497-4
Killiny N., S.E. Jones, Y. Nehela, F. Hijaz, M. Dutt, F.G. Gmitter and J.W. Grosser (2018) All roads lead to Rome: Towards understanding different avenues of tolerance to huanglongbing in citrus cultivars. Plant Physiology and Biochemistry129:1-10. https://doi.org/10.1016/j.plaphy.2018.05.005
Levy, A., El-Mohtar, C., Wang, C. et al. A new toolset for protein expression and subcellular localization studies in citrus and its application to citrus tristeza virus proteins. Plant Methods 14, 2 (2018). https://doi.org/10.1186/s13007-017-0270-7
Liu, Z., X. X. Ge, W. Qiu, J. M. Long, H. H. Jia, W. Yang, M. Dutt, X. M. Wu and W Guo. 2018. Overexpression of a B3 transcription factor CsFUS3 promotes somatic embryogenesis in Citrus. Plant Science 277: 121-131. https://doi.org/10.1016/j.plantsci.2018.10.015
Ma, H., Wang, M., Gai, Y., Fu, H., Zhang, B., Ruan, R., Chung, K., & Li, H. (2018). Thioredoxin and glutaredoxin systems required for oxidative stress resistance, fungicide sensitivity and virulence of Alternaria alternata. Applied and Environmental Microbiology, 84(14). https://doi.org/10.1128/AEM.00086-18
Niedz, R.P., Marutani-Hert, M. A filter paper-based liquid culture system for citrus shoot organogenesis—a mixture-amount plant growth regulator experiment. In Vitro Cell.Dev.Biol.-Plant 54, 658–671 (2018). https://doi.org/10.1007/s11627-018-9940-z
Omar, A.A., Murata, M.M., El-Shamy, H.A. et al. Enhanced resistance to citrus canker in transgenic mandarin expressing Xa21 from rice. Transgenic Res 27, 179–191 (2018). https://doi.org/10.1007/s11248-018-0065-2
Vu TX, Ngo TT, Mai LTD, Bui TT, Le DH, Bui HTV, Nguyen HQ, Ngo BX, Tran VT. A highly efficient Agrobacterium tumefaciens-mediated transformation system for the postharvest pathogen Penicillium digitatum using DsRed and GFP to visualize citrus host colonization. J Microbiol Methods. 2018 Jan;144:134-144. https://doi.org/10.1016/j.mimet.2017.11.019. Epub 2017 Nov 23.
2017
Acanda, Y., Canton, M., Wu, H., and Zale, J. (2017). Kanamycin selection in temporary immersion bioreactors allows visual selection of transgenic citrus shoots. Plant Cell Tissue and Organ Culture 129, 351-357.
De Francesco, A., Costa, N., and Garcia, M.L. (2017). Citrus psorosis virus coat protein-derived hairpin construct confers stable transgenic resistance in citrus against psorosis A and B syndromes. Transgenic Res. 26, 225-235.
Noelia Sendin, L., Georgina Orce, I., Liliana Gomez, R., Enrique, R., Grellet Bournonville, C.F., Sergio Noguera, A., Alberto Vojnov, A., Rosa Marano, M., Pedro Castagnaro, A., and Paula Filippone, M. (2017). Inducible expression of Bs2 R gene from Capsicum chacoense in sweet orange (Citrus sinensis L. Osbeck) confers enhanced resistance to citrus canker disease. Plant Mol. Biol. 93, 607-621.
Orbovic, V., Fields, J.S., and Syvertsen, J.P. (2017). Transgenic citrus plants expressing the p35 anti-apoptotic gene have altered response to abiotic stress. Horticulture Environment and Biotechnology 58, 303-309.
Shimada, T., Endo, T., Rodriguez, A., Fujii, H., Goto, S., Matsuura, T., Hojo, Y., Ikeda, Y., Mori, I.C., Fujikawa, T., Pena, L., and Omura, M. (2017). Ectopic accumulation of linalool confers resistance to Xanthomonas citri subsp citri in transgenic sweet orange plants. Tree Physiol. 37, 654-664.
Zhang, Y., Zhang, D., Zhong, Y., Chang, X., Hu, M., and Cheng, C. (2017). A simple and efficient in planta transformation method for pommelo (Citrus maxima) using Agrobacterium tumefaciens. Scientia Horticulturae 214, 174-179.
Zou, X., Jiang, X., Xu, L., Lei, T., Peng, A., He, Y., Yao, L., and Chen, S. (2017). Transgenic citrus expressing synthesized cecropin B genes in the phloem exhibits decreased susceptibility to Huanglongbing. Plant Mol. Biol. 93, 341-353.
2016
Boscariol-Camargo, R.L., Takita, M.A., and Machado, M.A. (2016). Bacterial resistance in AtNPR1 transgenic sweet orange is mediated by priming and involves EDS1 and PR2. Tropical Plant Pathology 41, 341-349.
Dutt, M., Erpen, L., Ananthakrishnan, G., Barthe, G.A., Brlansky, R.H., Maiti, I.B., and Grosser, J.W. (2016). Comparative expression analysis of five caulimovirus promoters in citrus. Plant Cell Tissue and Organ Culture 126, 229-238.
Dutt, M., Barthe, G., Irey, M., and Grosser, J. (2016). Transgenic Citrus Expressing an Arabidopsis NPR1 Gene Exhibit Enhanced Resistance against Huanglongbing (HLB; Citrus Greening) (vol 10, e0137134, 2015). Plos One 11, e0147657.
Hao, G., Pitino, M., Duan, Y., and Stover, E. (2016). Reduced Susceptibility to Xanthomonas citri in Transgenic Citrus Expressing the FLS2 Receptor From Nicotiana benthamiana. Mol. Plant-Microbe Interact. 29, 132-142.
Hao, G., Stover, E., and Gupta, G. (2016). Overexpression of a Modified Plant Thionin Enhances Disease Resistance to Citrus Canker and Huanglongbing (HLB). Frontiers in Plant Science 7, 1078.
Hu, W., Li, W., Xie, S., Fagundez, S., McAvoy, R., Deng, Z., and Li, Y. (2016). Kn1 gene overexpression drastically improves genetic transformation efficiencies of citrus cultivars. Plant Cell Tissue and Organ Culture 125, 81-91.
Jia, H., Orbovic, V., Jones, J.B., and Wang, N. (2016). Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccpthA4:dCsLOB1.3 infection. Plant Biotechnology Journal 14, 1291-1301.
Mcnellis, T., Gottwald, T., Sinn, J., and Orbovic, V. (2016). Phenotypic effects of anti-Candidatus Liberibacter asiaticus antibody expression in grapefruit. Phytopathology 106, 26-26.
Reyes, C.A., De Francesco, A., Ocolotobiche, E.E., Costa, N., and Garcia, M.L. (2016). Uncontrolled Citrus psorosis virus infection in Citrus sinensis transgenic plants expressing a viral 24K-derived hairpin that does not trigger RNA silencing. Physiol. Mol. Plant Pathol. 94, 149-155.
Wu, H., Acanda, Y., Jia, H., Wang, N., and Zale, J. (2016). Biolistic transformation of Carrizo citrange (Citrus sinensis Osb. x Poncirus trifoliata L. Raf.). Plant Cell Rep. 35, 1955-1962.
Yang, L., Hu, W., Xie, Y., Li, Y., and Deng, Z. (2016). Factors affecting Agrobacterium-mediated transformation efficiency of kumquat seedling internodal stem segments. Scientia Horticulturae 209, 105-112.
Zhang, X., Wang, W., Wang, M., Zhang, H., and Liu, J. (2016). The miR396b of Poncirus trifoliata Functions in Cold Tolerance by Regulating ACC Oxidase Gene Expression and Modulating Ethylene-Polyamine Homeostasis. Plant and Cell Physiology 57, 1865-1878.
2015
Alvarez-Gerding, X., Cortes-Bullemore, R., Medina, C., Romero-Romero, J.L., Inostroza-Blancheteau, C., Aquea, F., and Arce-Johnson, P. (2015). Improved Salinity Tolerance in Carrizo Citrange Rootstock through Overexpression of Glyoxalase System Genes. Biomed Research International
Alvarez-Gerding, X., Espinoza, C., Inostroza-Blancheteau, C., and Arce-Johnson, P. (2015). Molecular and physiological changes in response to salt stress in Citrus macrophylla W plants overexpressing Arabidopsis CBF3/DREB1A. Plant Physiology and Biochemistry 92, 71-80.
Cheng Chun-zhen, Yang Jia-wei, Yan Hu-bin, Bei Xue-jun, Zhang Yong-yan, Lu Zhi-ming, and Zhong Guang-yan. (2015). Expressing p20 hairpin RNA of Citrus tristeza virus confers Citrus aurantium with tolerance/resistance against stem pitting and seedling yellow CTV strains. Journal of Integrative Agriculture 14, 1767-1777.
Dutt, M., Barthe, G., Irey, M., and Grosser, J. (2015). Transgenic Citrus Expressing an Arabidopsis NPR1 Gene Exhibit Enhanced Resistance against Huanglongbing (HLB; Citrus Greening). Plos One 10, e0137134.
Orbovic, V., Grosser, J.W. (2015). Citrus Transformation Using Juvenile Tissue Explants. Agrobacterium Protocols, Volume 2, Third Edition 1224, 245-257.
Orbovic, V., Shankar, A., Peeples, M.E., Hubbard, C., and Zale, J. (2015). Citrus Transformation Using Mature Tissue Explants. Agrobacterium Protocols, Volume 2, Third Edition 1224, 259-273.
Peng, A., Xu, L., He, Y., Lei, T., Yao, L., Chen, S., and Zou, X. (2015). Efficient production of marker-free transgenic 'Tarocco' blood orange (Citrus sinensis Osbeck) with enhanced resistance to citrus canker using a Cre/loxP site-recombination system. Plant Cell Tissue and Organ Culture 123, 1-13.
Soler, N., Fagoaga, C., Lopez, C., Moreno, P., Navarro, L., Flores, R., and Pena, L. (2015). Symptoms induced by transgenic expression of p23 from Citrus tristeza virus in phloem-associated cells of Mexican lime mimic virus infection without the aberrations accompanying constitutive expression. Molecular Plant Pathology 16, 388-399.
Wu, H., Acanda, Y., Shankar, A., Peeples, M., Hubbard, C., Orbovic, V., and Zale, J. (2015). Genetic Transformation of Commercially Important Mature Citrus Scions. Crop Sci. 55, 2786-2797.
-
Literature 2014-2012
2014
Gong, X., Zhang, J., and Liu, J. (2014). A stress responsive gene of Fortunella crassifolia FcSISP functions in salt stress resistance. Plant Physiology and Biochemistry 83, 10-19.
Li Ding-li, Xiao Xuan, and Guo Wen-wu. (2014). Production of Transgenic Anliucheng Sweet Orange (Citrus sinensis Osbeck) with Xa21 Gene for Potential Canker Resistance. Journal of Integrative Agriculture 13, 2370-2377.
Ma YuanYuan, Zou XiuPing, Peng AiHong, Xu LanZhen, He YongRui, and Chen ShanChun. (2014). Ectopic expression analysis of Limonoid UDP-glucosyltransferase gene ( citLGT) in transgenic Citrus sinensis 'Jincheng'. Journal of Fruit Science 31, 181-186.
Muniz, F.R., Souza, A., Harakava, R., Alves Mourao Filho,Francisco de Assis, Stach-Machado, D.R., Rezende, J.A.M., Febres, V.J., Moore, G.A., and Mendes, B.M.J. (2014). Reaction of transgenic Citrus sinensis plants to Citrus tristeza virus infection by Toxoptera citricida. Eur. J. Plant Pathol. 139, 151-159.
Pinheiro, T.T., Figueira, A., and Latado, R.R. (2014). Early-flowering sweet orange mutant 'x11' as a model for functional genomic studies of Citrus. BMC Research Notes 7, 511-511.
Rodriguez, A., Shimada, T., Cervera, M., Alquezar, B., Gadea, J., Gomez-Cadenas, A., Jose De Ollas, C., Jesus Rodrigo, M., Zacarias, L., and Pena, L. (2014). Terpene Down-Regulation Triggers Defense Responses in Transgenic Orange Leading to Resistance against Fungal Pathogens. Plant Physiol. 164, 321-339.
Rossignol, P., Orbovic, V., and Irish, V.F. (2014). A dexamethasone-inducible gene expression system is active in Citrus plants. Scientia Horticulturae 172, 47-53.
Sun, L., Zhang, J., Mei, L., and Hu, C. (2014). Molecular cloning, promoter analysis and functional characterization of APETALA 1-like gene from precocious trifoliate orange (Poncirus trifoliata L. Raf.). Scientia Horticulturae 178, 95-105.
Xiao, X., Ma, F., Chen, C., and Guo, W. (2014). High efficient transformation of auxin reporter gene into trifoliate orange via Agrobacterium rhizogenes-mediated co-transformation. Plant Cell Tissue and Organ Culture 118, 137-146.
Zou, X., Song, E., Peng, A., He, Y., Xu, L., Lei, T., Yao, L., and Chen, S. (2014). Activation of three pathogen-inducible promoters in transgenic citrus (Citrus sinensis Osbeck) after Xanthomonas axonopodis pv. citri infection and wounding. Plant Cell Tissue and Organ Culture 117, 85-98.
2013
Alvarez, X., Medina, C., Cortes, R., Aquea, F., Rojas, S., and Arce-Johnson, P. (2013). Ex vitro multiplication of transgenic citrus rootstock plants carrying the CBF3 transcription factor gene from Arabidopsis thaliana. Acta Horticulturae 137-142.
An, C.F., Orbovic, V., and Mou, Z.L. (2013). An efficient intragenic vector for generating intragenic and cisgenic plants in citrus. American Journal of Plant Sciences 4, 2131-2137.
Arantes Felipe, R.T., Alves Mourao Filho,Francisco de Assis, Lopes, S.A., Januzzi Mendes, B.M., Behling, M., and Pereira Junior, E.V. (2013). Reaction of sweet orange cultivars expressing the attacin A gene to 'Candidatus Liberibacter asiaticus' infection. Pesquisa Agropecuaria Brasileira 48, 1440-1448.
Attilio, L.B., Alves Mourao Filho,Francisco de Assis, Harakava, R., da Silva, T.L., Miyata, L.Y., Liborio Stipp, L.C., and Januzzi Mendes, B.M. (2013). Genetic transformation of sweet oranges with the D4E1 gene driven by the AtPP2 promoter. Pesquisa Agropecuaria Brasileira 48, 741-747.
Bunnag, S., and Tangpong, D. (2013). Delivery of an antisense ACC oxidase gene into Citrus reticulata Blanco. mediated by Agrobacterium tumefaciens. AAB Bioflux 5, 29-38.
Chen, X., Barnaby, J.Y., Sreedharan, A., Huang, X., Orbovic, V., Grosser, J.W., Wang, N., Dong, X., and Song, W. (2013). Over-expression of the citrus gene CtNH1 confers resistance to bacterial canker disease. Physiol. Mol. Plant Pathol. 84, 115-122.
de Carvalho, K., Freitas de Campos, M.K., Domingues, D.S., Protasio Pereira, L.F., and Esteves Vieira, L.G. (2013). The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 40, 3269-3279.
Fu, X., and Liu, J. (2013). Transcriptional profiling of canker-resistant transgenic sweet orange (Citrus sinensis Osbeck) constitutively overexpressing a spermidine synthase gene. BioMed Research International 2013, 918136-918136.
Furman, N., Kobayashi, K., Cecilia Zanek, M., Calcagno, J., Laura Garcia, M., and Mentaberry, A. (2013). Transgenic sweet orange plants expressing a dermaseptin coding sequence show reduced symptoms of citrus canker disease. J. Biotechnol. 167, 412-419.
Gong, X., and Liu, J. (2013). Genetic transformation and genes for resistance to abiotic and biotic stresses in Citrus and its related genera. Plant Cell Tissue and Organ Culture 113, 137-147.
Hu Wei, Yang Li, Xie YuMing, Zhou Xiao, Ge HongJuan, Li DaZhi, and Deng ZiNiu. (2013). Further study on improving genetic transformation efficiency of succari orange. Journal of Hunan Agricultural University 39, 371-376.
Jin, S.B., Sun, H.J., Al Bachchu, M.A., Chung, S.J., Lee, J., Han, S., Yun, J.H., Boo, K.W., Lee, D., Riu, K.Z., and Kim, J. (2013). Production of Recombinant Miraculin Protein Using Transgenic Citrus Cell Suspension Culture System. Journal of the Korean Society for Applied Biological Chemistry 56, 271-274.
Lu RongSheng, Han MeiLi, Lin Rui, Ma YueFeng, Yang YuXia, and Qing JianLin. (2013). Study on influencing factors for transformation of Citrus paradisi mediated by Agrobacterium. Guizhou Agricultural Sciences 15-19.
Orbovic, V., Goellner, E.M., and Soria, P. (2013). The effect of arabinogalactan proteins on regeneration potential of juvenile citrus explants used for genetic transformation by Agrobacterium tumefaciens. Acta Physiologiae Plantarum 35, 1409-1419.
Peixoto de Oliveira, M.L., de Lima Silva, C.C., Abe, V.Y., Cardoso Costa, M.G., Cernadas, R.A., and Benedetti, C.E. (2013). Increased Resistance Against Citrus Canker Mediated by a Citrus Mitogen-Activated Protein Kinase. Mol. Plant-Microbe Interact. 26, 1190-1199.
Wang, H., Petri, C., Burgos, L., and Alburquerque, N. (2013). Phosphomannose-isomerase as a selectable marker for transgenic plum (Prunus domestica L.). Plant Cell Tissue and Organ Culture 113, 189-197.
Zou, X., Peng, A., Xu, L., Liu, X., Lei, T., Yao, L., He, Y., and Chen, S. (2013). Efficient auto-excision of a selectable marker gene from transgenic citrus by combining the Cre/loxP system and ipt selection. Plant Cell Rep. 32, 1601-1613.
2012
Caruso, P., Baldoni, E., Mattana, M., Paolo, D.P., Genga, A., Coraggio, I., Russo, G., Picchi, V., Recupero, G.R., and Locatelli, F. (2012). Ectopic expression of a rice transcription factor, Mybleu, enhances tolerance of transgenic plants of Carrizo citrange to low oxygen stress. Plant Cell Tissue and Organ Culture 109, 327-339.
Cevik, B., Lee, R.F., and Niblett, C.L. (2012). Agrobacterium-mediated transformation of grapefruit with the wild-type and mutant RNA-dependent RNA polymerase genes of Citrus tristeza virus. Turkish Journal of Agriculture and Forestry 36, 195-206.
Curtis, I.S., and Mirkov, T.E. (2012). Influence of surfactants on growth and regeneration from mature internodal stem segments of sweet orange (Citrus sinensis) cv. Hamlin. Plant Cell Tissue and Organ Culture 108, 345-352.
Dutt, M., Ananthakrishnan, G., Jaromin, M.K., Brlansky, R.H., and Grosser, J.W. (2012). Evaluation of four phloem-specific promoters in vegetative tissues of transgenic citrus plants. Tree Physiol. 32, 83-93.
Favero, P., Alves Mourao Filho,Francisco de Assis, Liborio Stipp, L.C., and Januzzi Mendes, B.M. (2012). Genetic transformation of three sweet orange cultivars from explants of adult plants. Acta Physiologiae Plantarum 34, 471-477.
Khan, E.U., Fu, X., and Liu, J. (2012). Agrobacterium-mediated genetic transformation and regeneration of transgenic plants using leaf segments as explants in Valencia sweet orange. Plant Cell Tissue and Organ Culture 109, 383-390.
Koh, E., Zhou, L., Williams, D.S., Park, J., Ding, N., Duan, Y., and Kang, B. (2012). Callose deposition in the phloem plasmodesmata and inhibition of phloem transport in citrus leaves infected with "Candidatus Liberibacter asiaticus". Protoplasma 249, 687-697.
Li, Z.T., Gmitter, F.G., Jr., Grosser, J.W., Chen, C., and Gray, D.J. (2012). Isolation and characterization of a novel anthocyanin-promoting MYBA gene family in Citrus. Tree Genetics & Genomes 8, 675-685.
Mondal, S.N., Dutt, M., Grosser, J.W., and Dewdney, M.M. (2012). Transgenic citrus expressing the antimicrobial gene Attacin E (attE) reduces the susceptibility of 'Duncan' grapefruit to the citrus scab caused by Elsinoe fawcettii. Eur. J. Plant Pathol. 133, 391-404.
Muniz, F.R., De Souza, A.J., Stipp, L.C.L., Schinor, E., Freitas, W., Jr., Harakava, R., Stach-Machado, D.R., Rezende, J.A.M., Mourao Filho, F.A.A., and Mendes, B.M.J. (2012). Genetic transformation of Citrus sinensis with Citrus tristeza virus (CTV) derived sequences and reaction of transgenic lines to CTV infection. Biol. Plant. 56, 162-166.
Sendin, L.N., Filippone, M.P., Orce, I.G., Rigano, L., Enrique, R., Pena, L., Vojnov, A.A., Marano, M.R., and Castagnaro, A.P. (2012). Transient expression of pepper Bs2 gene in Citrus limon as an approach to evaluate its utility for management of citrus canker disease. Plant Pathol. 61, 648-657.
Singer, S.D., and Cox, K.D. (2012). The CsSUT1 promoter from Citrus sinensis confers sink-specific expression of a downstream reporter gene in transgenic Arabidopsis. Journal of Plant Biochemistry and Biotechnology 21, 167-172.
Soler, N., Plomer, M., Fagoaga, C., Moreno, P., Navarro, L., Flores, R., and Pena, L. (2012). Transformation of Mexican lime with an intron-hairpin construct expressing untranslatable versions of the genes coding for the three silencing suppressors of Citrus tristeza virus confers complete resistance to the virus. Plant Biotechnology Journal 10, 597-608.
Wen, L., Tan, B., and Guo, W. (2012). Estimating transgene copy number in precocious trifoliate orange by TaqMan real-time PCR. Plant Cell Tissue and Organ Culture 109, 363-371.
-
Literature 2011-2007
2011
Al Bachchu MA, Jin SB, Park JW, Boo KH, Sun HJ, Kim YW, Lee HY, Riu KZ, Kim JH. Functional Expression of Miraculin, a Taste-modifying Protein, in Transgenic Miyagawa Wase Satsuma Mandarin (Citrus unshiu Marc.). Journal of the Korean Society for Applied Biological Chemistry 2011;54(1):24-29.
de Campos MKF, de Carvalho K, de Souza FS, Marur CJ, Pereira LFP, Bespalhok JC, Vieira LGE. Drought tolerance and antioxidant enzymatic activity in transgenic 'Swingle' citrumelo plants over-accumulating proline. Environmental and Experimental Botany 2011;72(2):242-250.
Dutt M, Vasconcellos M, Grosser JW. Effects of antioxidants on Agrobacterium-mediated transformation and accelerated production of transgenic plants of Mexican lime (Citrus aurantifolia Swingle). Plant Cell Tissue and Organ Culture 2011;107(1):79-89.
Fan, J., Liu, X., Xu, S., Xu, Q., and Guo, W. (2011). T-DNA direct repeat and 35S promoter methylation affect transgene expression but do not cause silencing in transgenic sweet orange. Plant Cell Tissue and Organ Culture 107, 225-232.
Fu, X., Khan, E.U., Hu, S., Fan, Q., and Liu, J. (2011). Overexpression of the betaine aldehyde dehydrogenase gene from Atriplex hortensis enhances salt tolerance in the transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Environ. Exp. Bot. 74, 106-113.
He YR, Chen SC, Peng AH, Zou XP, Xu LZ, Lei TG, Liu XF, Yao LX. Production and evaluation of transgenic sweet orange (Citrus sinensis Osbeck) containing bivalent antibacterial peptide genes (Shiva A and Cecropin B) via a novel Agrobacterium-mediated transformation of mature axillary buds. Scientia Horticulturae 2011;128(2):99-107.
Loeza-Kuk E, Gutierrez-Espinosa MA, Ochoa-Martinez DL, Villegas-Monter A, Mora-Aguilera G, Palacios-Torres EC, Perez-Molphe-Balch E. RESISTANCE ANALYSIS IN GRAPEFRUIT AND MEXICAN LIME TRANSFORMED WITH the p25 Citrus tristeza virus GEN. Agrociencia 2011;45(1):55-65.
Machado, M.A., Cristofani-Yaly, M., and Bastianel, M. (2011). Breeding, Genetic and Genomic of Citrus for Disease Resistance. Revista Brasileira De Fruticultura 33, 158-172.
Miyata LY, Mourao FDA, Scarpare JA, Zambon F, Bassan MM, Mendes BMJ, Harakava R. GENETIC TRANSFORMATION EFFICIENCY OF 'CARRIZO' CITRANGE WITH TWO GENE CONSTRUCTIONS. Revista Brasileira De Fruticultura 2011;33(1):311-315.
Orbovic V, Dutt M, Grosser JW. Seasonal effects of seed age on regeneration potential and transformation success rate in three citrus cultivars. Scientia Horticulturae 2011;127(3):262-266.
Orbovic V, Soria P, Moore GA, Grosser JW. The use of citrus tristeza virus (CTV) containing a green fluorescent protein gene as a tool to evaluate resistance/tolerance of transgenic citrus plants. Crop Protection 2011;30(5):572-576.
Rattanpal, H.S., Kaur, G., and Gupta, M. (2011). In vitro plant regeneration in rough lemon (Citrus jambhiri Lush) by direct organogenesis. African Journal of Biotechnology 10, 13724-13728.
Reyes CA, De Francesco A, Pena EJ, Costa N, Plata MI, Sendin L, Castagnaro AP, Garcia ML. Resistance to Citrus psorosis virus in transgenic sweet orange plants is triggered by coat protein-RNA silencing. Journal of Biotechnology 2011;151(1):151-158.
Reyes CA, Zanek MC, Velazquez K, Costa N, Plata MI, Garcia ML. Generation of Sweet Orange Transgenic Lines and Evaluation of Citrus psorosis virus-derived Resistance against Psorosis A and Psorosis B. Journal of Phytopathology 2011;159(7-8):531-537.
Singer SD, Hily JM, Cox KD. The sucrose synthase-1 promoter from Citrus sinensis directs expression of the beta-glucuronidase reporter gene in phloem tissue and in response to wounding in transgenic plants. Planta 2011;234(3):623-637.
Xu SX, Cai XD, Tan B, Guo WW. Comparison of expression of three different sub-cellular targeted GFPs in transgenic Valencia sweet orange by confocal laser scanning microscopy. Plant Cell Tissue and Organ Culture 2011;104(2):199-207.
Xu SX, Cai XD, Tan B, Li DL, Guo WW. Effect of ploidy increase on transgene expression: example from Citrus diploid cybrid and allotetraploid somatic hybrid expressing the EGFP gene. Protoplasma 2011;248(3):531-540.
Yang L, Hu CH, Li N, Zhang JY, Yan JW, Deng ZN. Transformation of sweet orange Citrus sinensis (L.) Osbeck with pthA-nls for acquiring resistance to citrus canker disease. Plant Molecular Biology 2011;75(1-2):11-23.
2010
Ballester A, Cervera M, Pena L. Selectable marker-free transgenic orange plants recovered under non-selective conditions and through PCR analysis of all regenerants. Plant Cell Tissue and Organ Culture 2010;102(3):329-336.
Cardoso SC, Barbosa-Mendes JM, Boscariol-Camargo RL, Christiano RSC, Bergamin A, Vieira MLC, Mendes BMJ, Mourao FDA. Transgenic Sweet Orange (Citrus sinensis L. Osbeck) Expressing the attacin A Gene for Resistance to Xanthomonas citri subsp citri. Plant Molecular Biology Reporter 2010;28(2):185-192.
Cervera M, Esteban O, Gil M, Gorris MT, Martinez MC, Pena L, Cambra M. Transgenic expression in citrus of single-chain antibody fragments specific to Citrus tristeza virus confers virus resistance. Transgenic Research 2010;19(6):1001-1015.
Dutt M, Grosser JW. An embryogenic suspension cell culture system for Agrobacterium-mediated transformation of citrus. Plant Cell Reports 2010;29(11):1251-1260.
Dutt M, Lee DH, Grosser JW. Bifunctional selection-reporter systems for genetic transformation of citrus: mannose- and kanamycin-based systems. In Vitro Cellular & Developmental Biology-Plant 2010;46(6):467-476.
Dutt M, Madhavaraj J, Grosser JW. Agrobacterium tumefaciens-mediated genetic transformation and plant regeneration from a complex tetraploid hybrid citrus rootstock. Scientia Horticulturae 2010;123(4):454-458.
Li ZM, Zhang JZ, Mei L, Deng XX, Hu CG, Yao JL. PtSVP, an SVP homolog from trifoliate orange (Poncirus trifoliata L. Raf.), shows seasonal periodicity of meristem determination and affects flower development in transgenic Arabidopsis and tobacco plants. Plant Molecular Biology 2010;74(1-2):129-142.
Lopez C, Cervera M, Fagoaga C, Moreno P, Navarro L, Flores R, Pena L. Accumulation of transgene-derived siRNAs is not sufficient for RNAi-mediated protection against Citrus tristeza virus in transgenic Mexican lime. Molecular Plant Pathology 2010;11(1):33-41.
Mendes BMJ, Cardoso SC, Boscariol-Camargo RL, Cruz RB, Mourao FAA, Bergamin A. Reduction in susceptibility to Xanthomonas axonopodis pv. citri in transgenic Citrus sinensis expressing the rice Xa21 gene. Plant Pathology 2010;59(1):68-75.
Nishikawa F, Endo T, Shimada T, Fujii H, Shimizu T, Kobayashi Y, Araki T, Omura M. Transcriptional changes in CiFT-introduced transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Tree Physiology 2010;30(3):431-439.
Wang J, Liu JH, Kurosawa T, Nada K, Ban Y, Moriguchi T. Cloning, biochemical identification, and expression analysis of a gene encoding S-adenosylmethionine decarboxylase in navel orange (Citrus sinensis Osbeck). Journal of Horticultural Science & Biotechnology 2010;85(3):219-226.
Zhang XD, Francis MI, Dawson WO, Graham JH, Orbovic V, Triplett EW, Mou ZL. Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. European Journal of Plant Pathology 2010;128(1):91-100.
2009
Barbosa-Mendes JM, Mourao FDA, Bergamin A, Harakava R, Beer SV, Mendes BMJ. Genetic transformation of Citrus sinensis cv. Hamlin with hrpN gene from Erwinia amylovora and evaluation of the transgenic lines for resistance to citrus canker. Scientia Horticulturae 2009;122(1):109-115.
Cervera M, Navarro L, Pena L. Gene stacking in 1-year-cycling APETALA1 citrus plants for a rapid evaluation of transgenic traits in reproductive tissues. Journal of Biotechnology 2009;140(3-4):278-282.
de Oliveira MLP, Febres VJ, Costa MGC, Moore GA, Otoni WC. High-efficiency Agrobacterium-mediated transformation of citrus via sonication and vacuum infiltration. Plant Cell Reports 2009;28(3):387-395.
Deng W, Luo KM, Li ZG, Yang YW, Hu N, Wu Y. Overexpression of Citrus junos mitochondrial citrate synthase gene in Nicotiana benthamiana confers aluminum tolerance. Planta 2009;230(2):355-365.
Dutt M, Grosser JW. Evaluation of parameters affecting Agrobacterium-mediated transformation of citrus. Plant Cell Tissue and Organ Culture 2009;98(3):331-340.
Koca U, Berhow MA, Febres VJ, Champ KI, Carrillo-Mendoza O, Moore GA. Decreasing unpalatable flavonoid components in Citrus: the effect of transformation construct. Physiologia Plantarum 2009;137(2):101-114.
Li DL, Tan B, Duan YX, Guo WW. Regeneration of transgenic citrus plants from the trimmed shoot/root region of etiolated seedlings. Biologia Plantarum 2009;53(3):578-582.
Pasquali G, Orbovic V, Grosser J. Transgenic grapefruit plants expressing the P(APETALA3)-IPT (gp) gene exhibit altered expression of PR genes. Plant Cell Tissue and Organ Culture 2009;97(2):215-223.
Tan B, Li DL, Xu SX, Fan GE, Fan J, Guo WW. Highly efficient transformation of the GFP and MAC12.2 genes into precocious trifoliate orange (Poncirus trifoliata L. Raf), a potential model genotype for functional genomics studies in Citrus. Tree Genetics & Genomes 2009;5(3):529-537.
Tong Z, Tan B, Zhang JC, Hu ZY, Guo WW, Deng XX. Using precocious trifoliate orange (Poncirus trifoliata L. Raf.) to establish a short juvenile transformation platform for citrus. Scientia Horticulturae 2009;119(3):335-338.
Wang CX, Hong N, Wang GP, Jiang B, Fan XD. Effects of Citrus tristeza virus on the growth of in vitro-cultured citrus. Journal of Plant Pathology 2009;91(2):357-363.
2008
Ananthakrishnan, G., Orbovic, V., Pasquali, G., Alovi, M., & Grosser, J. W. (2008). Transfer of citrus tristeza virus (CTV)-derived resistance candidate sequences to four grapefruit cultivars through Agrobacterium-mediated genetic transformation. In vitro cellular & developmental biology - plant, 43 (6), 593-601.
Ballester, A., Cervera, M., & Pena, L. (2008). Evaluation of selection strategies alternative to nptII in genetic transformation of citrus. Plant Cell Reports, 27 (6), 1005-1015.
Cervera, M., Navarro, A., Navarro, L., & Pena, L. (2008). Production of transgenic adult plants from clementine mandarin by enhancing cell competence for transformationa nd regeneration. Tree Physiology, 28 (1), 55-66.
Febres, V. J., Lee, R. F., & Moore, G. A. (2008) Transgenic resistance to Citrus tristeza virus in grapefruit. Plant Cell Reports, 27 (1), 93-104.
Guo, W. W., Duan, Y. X., Li, D. L., Liu, X., Tan, B., Cai, X. D., Grosser, J. W., Deng, X. X. (2008). Citrus genetic transformation with interest target genes and further uses of transgenic lines in somatic fusion. Acta Horticulturae, 773, 25-28.
Omar, A. A., Dekkers, M.G.H., Graham, J.H., & Grosser, J.W. (2008). Estimation of transgene copy number in transformed citrus plants by auantitative multiplex real-time PCR. Biotechnology Progress, 24 (6), 1241-1248.
Rodriguez, A., Cervera, M., Peris, J. E., & Pena, L. (2008). The same treatment for transgenic shoot regeneration elicits the opposite effect in mature explants from two closely related sweet orange (Citrus sinensis (L.) Osb.) genotypes. Plant Cell, Tissue, and Organ Culture, 93 (1), 97-106.
Zanek, M. C., Reyes, C. A., Cervera, M., Pena, E. J., Velazquez, K., Costa, N., Plata, M. I., Grau, O., Pena, L., Garcia, M. L. (2008). Genetic transformation of sweet orange with the coat protein gene of citrus psorosis virus and evaluation of resistance against the virus. Plant Cell Reports 27 (1), 57-66.
Zou, XiuPing, Li, DeMou, Ying, Luo Xiao, KeMing, Luo, & Yan, Pei. (2008). An improved procedure for Agobacterium-mediated transformation of trifoliate orange (Poncirus trifoliata L. Raf.) via indirect organogenesis. In Vitro Cellular & Developmental Biology - Plant, 44 (3), 169-177.
2007
Ballester, A., Cervera, M., & Pena, L. (2007). Prodcution of transgneic citrus plants using isopenenyltransferase (ipt) positive selection and removal of the marker gene by site-specific recombination. Acta Horticulturae, 738, 191-200.
Ballester, A., Cervera, M., & Pena, L. (2007). Efficient production of transgenic citrus plants using isopentenyl transferase positive selectiona nd removal of the marker gene by site-specific recombination. Plant Cell Reports, 26 (1), 39-45.
Chen, C. X., Zheng, Q., Soneji, J. R., Xiang, X., Choi, Y. A., Huang, S., Rao, M. N., & Gmitter, F. G., Jr. (2007). Agrobacterium-mediated transformation in citrus using a new GFP vector. Acta Horticulturae, 738, 207-211.
Chen, C. X., Zheng, Q., Xiang, X., Soneji, J. R., Huang, S., Choi, Y. A., Rao, M. N., & Gmitter, F. G., Jr. (2007). Development of pGreen-derived GFP binary vectors for citrus transformation. HortScience, 42 (1), 7-10.
Omar, A. A., Song, W. Y., & Grosser, J. W. (2007). Introduction of Xa21, a Xanthomonas-resistance gene from rice, into 'Hamlin' sweet orange [Citrus sinensis (L.) Osbeck] using protoplast-GFP co-transformation or single plasmid transformation. Journal of Horticultural Science and Biotechnology, 82 (6), 914-923.
Omar, A. A., & Grosser, J. W. (2007). Protoplast co-transformationa nd regeneration of transgenic 'Hamlin' sweet orange plants containing a cDNA Xa21 Xanthomonas resistance gene and GFP. Acta Horticulturae, 738, 235-243
Orbovic, V., Pasquali, G., & Grosser, J. W. (2007). A GFP-containing binary vector for Agrobacterium tumefaciens-mediated plant transformation. Acta Horticulturae, 738, 245-253.
Soneji, J. R., Chen, C. X., Rao, M. N., Huang, S., Choi, Y. A., & Gmitter, F. G., Jr. (2007). Agrobacterium, mediated transformation of citrus using two binary vectors. Acta Horticulturae, 738, 261-264.
-
Literature 2006-2003
2006
Azevedo, F. A., Mourao Filho, F.A.A., Mendes, B. M. J., Almeida, W. A. B., Schinor, E. H., Pio, r., Barbos, J. M., Guidetti-Gonzalez, S., Carrer, H., & Lam, E. (2006). Genetic transformation of Rangpur lime (Citrus limonia Osbeck) with the bO (bacterio-opsin) gene and its initial evaluation for Phytophthora nicotianae resistance. Plant Molecular Biology Reporter, 24 (2), 185-196.
Boscariol, R. L., Monteiro, M., Takahashi, E. K., Chabregas, S. M., Vieira, M. L. C., Vieira, L. G. E., Pereira, L. F. P., Mourao Filho, F. de A. A., Cardoso, S. C., Christiano, R. S. C., Bergamin Filho, A., Barbosa, J. M., Azevedo, F. A., & Mendes, B. M. J. (2006). Attacin A gene from Tricloplusia ni reduces susceptibility to Xanthomonas axonopodis pv. citri in transgenic Citrus sinensis 'Hamlin'. Journal of the American Society for Horticultural Science, 131 (4), 530-536.
Cai, XiaoDong, Liu, Xin, & Guo WenWu. (2006). GFP expression as an indicator of somatic hybrids between transgenic Satsuma mandarin and calamondin at embryoid stage. Plant Cell, Tissue, and Organ Culture, 87 (3), 245-253.
Fagoaga, C., Lopez, C., Mendoza, A. H. de, Moreno, P., Navarro, L., Flores, R., & Pena, L. (2006). Post-trnascriptional gene silencing of the p23 silencing suppressor of Citrus tristeza virus confers resistance ot the virus in transgenic Mexican lime. Plant Molecular Biology, 60 (2), 153-165.
Khawale, R. N., Singh, S. K., Garg, G., Baranwal, V. K., & Ajirlo, S. A. (2006). Agrobacterium-mediated genetic transformation of Nagpur mandarin (Citrus reticulata Blando. Current Science, 91 (12), 1700-1705.
2005
Cervera, M., Juarez, J., Navarro, L., & Pena, L. (2005). Genetic transformation of mature citrus plants. In Methods in Molecular Biology (pp. 177-187).
Endo, T., Shimada, T., Fujii, H., Kobayashi, Y., Araki, T., & Omura, M. (2005). Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Research, 14 (5), 703-712.
Fagoaga, C., Lopez, C., Moreno, P., Navarro, L., Flores, R., & Pena, L. (2005). Viral-like symptoms induced by the ectopic expression of the p23 gene of Citrus tristeza virus are citrus specific and do not correlate with the pathogenicity of the virus strain. Molecular Plant-Microbe Interactions, 18 (5), 435-445.
Guo, W. W., Duan, Y. X., Olivares-Fuster, O., Wu, Z. C., Arias, C. R., Burns, J. K., et al. (2005). Protoplast transformation and regeneration of transgenic Valencia sweet orange plants containing a juice quality-related pectin methylesterase gene. Plant Cell Reports, 24 (8), 482-486.
Guo, W. W., & Grosser, J. W. (2005). Somatic hylbrid vigor in Citrus: Direct evidence from protoplast fusion of an embryogenic callus line with a transgenic mesophyll parent expressing the GFP gene. Plant Science, 168 (6), 1541-1545.
Han, S. H., Ahn, H. J., Kang, S. K., & Kim, H. Y. (2005). Expression of green fluorescent protein gene in the callus of Satsuma mandarin (Citrus unshiu cv. Miyagawa Wase) by Agrobacterium-mediated transformation. Journal of the Korean Society for Horticultural Science, 46 (1), 39-42.
Kayim, M., Ceccardi, T. L., Berretta, M. J. G., Barthe, G. A., & Derrick, K. S. (2004). Introduction of a citrus blight-associated gene into Carrizo citrange [Citrus sinensis (L.) Osbc. x Poncirus trifoliata (L.) Raf.] by Agrobacterium-mediated transformation. Plant Cell Reports, 23 (6), 377-385.
Kayim, M., & Koc, N. K. (2005). Improved transformation efficiency in citrus by plasmolysis treatment. Journal of Plant Biochemistry and Biotechnology, 14 (1), 15-20.
Molinari, H. B. C., Marur, C. J., Bespalhok, J. C., Kobayashi, A. K., Pileggi, M., Leite, R. P., et al. (2004). Osmotic adjustment in transgenic citrus rootstock Carrizo citrange (Citrus sinensis Osb. x Poncirus trifoliata L. Raf.) overproducing proline. Plant Science, 167 (6), 1375-1381.
Rhim, S. L., Kim, I. I. G., Jin, T. E., Lee, J. H., Kuo, C. I., Suh, S. C., et al. (2004). Transformation of citrus with coleopteran specific delta-endotoxin gene from Bacillus thuringiensis ssp tenebrionis. Journal of Plant Biotechnology, 6 (1), 21-24.
Trainin, T., Lipsky, A., Levy, A. A., & Holland , D. (2005). Prolonged somatic transposition in citrus: The autonomous Ac transposable element remains active in the citrus genome for several years. Journal of the American Society for Horticultural Science, 130 (1), 95-101.
2004
Gentile, A., Z.N. Deng, S.l. Malfa, F. Domina, C. Germana, and E. Tribulato. 2004. Morphological and physiological effects of rolABC genes into citrus genome. Acta Horticulturae. 632: 235-242.
Hu, R., H. Wei, S. Chen, and Y. He. 2004. Construction of the plant expression vector with hepatitis A capsid protein fusion gene and genetic transformation of Citrus sinensis Osbeck. Hereditas Beijing. 26: 425-431.
Iwanami, T., T. Shimizu, T. Ito, and T. Hirabayashi. 2004. Tolerance to citrus mosaic virus in transgenic trifoliate orange lines harboring capsid polyprotein gene. Plant Disease. 88: 865-868.
Molinari, H.B.C., J.C. Bespalhok, A.K. Kobayashi, L.F.P. Pereira, and L.G.E. Vieira. 2004. Agrobacterium tumefaciens-mediated transformation of Swingle citrumelo (Citrus paradisi Macf. x Poncirus trifoliata L. Raf.) using thin epicotyl sections. Scientia Horticulturae. 99: 379-385.
Pena, L., R.M. Perez, M. CERVERA, J.A. JUAREZ, and L. NAVARRO. 2004. Early Events in Agrobacterium-mediated Genetic Transformation of Citrus Explants. Annals of Botany. 94: 67-74.
2003
Boscariol, R.L., W.A.B. Almeida, M.T.V.C. Derbyshire, F.A.A. Mourao-Filho, and B.M.J. Mendes. 2003. The use of the PMI/mannose selection system to recover transgenic sweet orange plants (Citrus sinensis L. Osbeck). Plant Cell Reports. 22: 122-128.
Li, D.D., W. Shi, and X.X. Deng. 2003. Factors influencing Agrobacterium-mediated embryogenic callus transformation of Valencia sweet orange (Citrus sinensis) containing the pTA29-barnase gene. Tree Physiology. 23: 1209-1215.
Niedz, R.P., W.L. McKendree, and R.G. Shatters. 2003. Electroporation of embryogenic protoplasts of sweet orange (Citrus sinensis (L.) Osbeck) and regeneration of transformed plants. In Vitro Cellular & Developmental Biology-Plant. 39: 586-594.
Olivares-Fuster, O., G.H. Fleming, M.R. Albiach-Marti, S. Gowda, W.O. Dawson, and J.W. Grosser. 2003. Citrus tristeza virus (CTV) resistance in transgenic citrus based on virus challenge of protoplasts. In Vitro Cellular & Developmental Biology-Plant. 39: 567-572.
Sambade, A., C. Lopez, L. Rubio, R. Flores, J. Guerri, and P. Moreno. 2003. Polymorphism of a specific region in gene p23 of Citrus tristeza virus allows discrimination between mild and severe isolates. Archives of Virology. 148: 2325-2340.



