Early Detection Techniques
The snaphots on this page describe methods currently under development that could detect HLB in the infection process (weeks or a few months, rather than years).
-
Metabolite changes in the tree can help us detect Huanglongbing
Research by Dr. Carolyn Slupsky, University of California, Davis
Article written by Carolyn Slupsky, Elizabeth Chin, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 9, 2025.What is the technique?
Metabolism is all of the processes an organism carries out in order to stay alive. There are two types of molecules involved in an organism’s metabolism: proteins and small molecular weight chemicals called metabolites. These molecules change in abundance when the metabolism of a living organism, such as a human, insect, or tree, is altered. Measuring these molecules provides a ‘snapshot’ of an organism’s metabolism. During infection, an organism’s metabolism is busy responding to the pathogen, as it works to protect itself. Thus, the ‘metabolite profile’ is different for healthy versus infected organisms. Carolyn Slupsky is developing methods to measure metabolite profiles of citrus trees, and to identify one that indicates early CLas (the bacteria that causes HLB) infection.
How can metabolites be used to identify infected trees?
Early detec
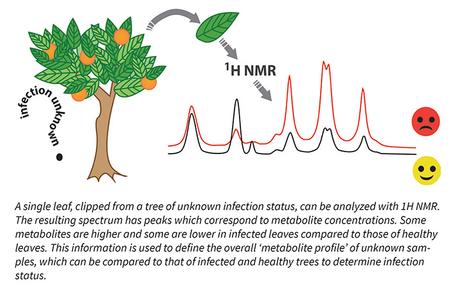 tion of CLas infection is critical to removing infected citrus trees and reducing HLB spread. The plant response to CLas occurs throughout the plant soon after infection, even before symptoms of HLB appear in leaves, twigs, fruit or roots. The changes in metabolite composition can be measured and used to identify infected trees. The Slupsky Lab uses a technique called 1H NMR (“proton NMR”) spectroscopy to measure these changes in metabolites. Leaves are dried and ground, and metabolites are extracted using specific chemicals. The resulting extract is run on the NMR, and the output- called a ‘spectrum’- is analyzed on a computer. Each spectrum has many peaks, and each peak or group of peaks corresponds to specific metabolites. This includes sugars (e.g., glucose and sucrose), amino acids (the building blocks of proteins), and other small molecules. The area under each peak provides information about the metabolite concentration, so the pattern of metabolite peaks during CLas infection defines the metabolite profile (Fig. 1). Metabolite profiles of unknown leaf samples can be compared to known profiles of CLas-infected and non-infected leaves. Then, unknown leaf samples can be categorized as infected or non-infected. This method, requiring only one leaf per tree, allows for rapid and noninvasive sampling.
tion of CLas infection is critical to removing infected citrus trees and reducing HLB spread. The plant response to CLas occurs throughout the plant soon after infection, even before symptoms of HLB appear in leaves, twigs, fruit or roots. The changes in metabolite composition can be measured and used to identify infected trees. The Slupsky Lab uses a technique called 1H NMR (“proton NMR”) spectroscopy to measure these changes in metabolites. Leaves are dried and ground, and metabolites are extracted using specific chemicals. The resulting extract is run on the NMR, and the output- called a ‘spectrum’- is analyzed on a computer. Each spectrum has many peaks, and each peak or group of peaks corresponds to specific metabolites. This includes sugars (e.g., glucose and sucrose), amino acids (the building blocks of proteins), and other small molecules. The area under each peak provides information about the metabolite concentration, so the pattern of metabolite peaks during CLas infection defines the metabolite profile (Fig. 1). Metabolite profiles of unknown leaf samples can be compared to known profiles of CLas-infected and non-infected leaves. Then, unknown leaf samples can be categorized as infected or non-infected. This method, requiring only one leaf per tree, allows for rapid and noninvasive sampling.Who is working on this project?
Carolyn Slupsky, a Professor in the Department of Food Science and Technology at the University of California, Davis, and her research team are using these methods to establish and validate metabolite-based markers for early detection of HLB.
What are the challenges and opportunities?
The main challenge is field validation, because truly healthy, non-CLas-infected trees are not guaranteed in the field if the psyllid vector is present. However, this method has been successful in correctly identifying CLas-infected field trees in Texas, and more field trials using screen-protected healthy control trees are underway. Preliminary data shows that the metabolite profiles of leaves infected with citrus tristeza virus (CTV), canker, or citrus stubborn infections are different from the metabolite profile of leaves infected with CLas. Although sampling is as easy as clipping one leaf from a tree, analysis of the NMR spectrum is currently a bottleneck because this method is only semi-automated and requires lab researcher input. Automation of NMR spectral analysis would help turn this into a high-throughput method.
Watch video about HLB, created by Dr. Slupsky’s lab.
https://youtu.be/dzKIigG24uEFunding source: This project is funded by the Citrus Research Board and a USDA-NIFA Hatch grant.
-
The ‘nose’ knows: Using citrus odor for early detection of HLB
Research by Dr. Cristina Davis, University of California, Davis
Article written by Cristina Davis, Mitchell McCartney, Elizabeth Grafton-Cardwell & Peggy G. Lemaux.
Revised 7/8/2025What is the technique?
Citrus trees emit volatile organic compounds (VOCs), or odors, that
are the endproducts of plant metabolis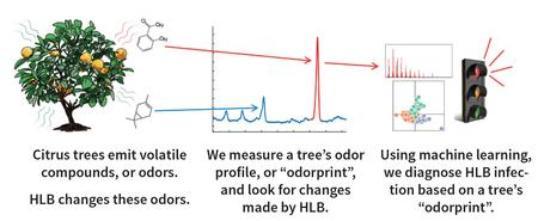 m. Researchers can measure the VOCs emitted by a plant and create an “odorprint”. Pathogens, such as the bacterium CLas that causes huanglongbing (HLB), affect plant metabolism and alter the odor profile of the plant. Thus, the odor profile of an HLB-infected tree is different from a healthy one. Because VOCs are emitted by the citrus tree as a whole, this method of detecting the bacterium can reveal infected trees earlier than PCR, a biochemical test that only reveals the disease if the specific plant part collected
m. Researchers can measure the VOCs emitted by a plant and create an “odorprint”. Pathogens, such as the bacterium CLas that causes huanglongbing (HLB), affect plant metabolism and alter the odor profile of the plant. Thus, the odor profile of an HLB-infected tree is different from a healthy one. Because VOCs are emitted by the citrus tree as a whole, this method of detecting the bacterium can reveal infected trees earlier than PCR, a biochemical test that only reveals the disease if the specific plant part collected
(leaf, twig or root) has the bacterium in it.Methods to measure VOCs
The Davis laboratory h
 as developed two volatile-based methods to detect HLB in citrus trees. Both methods provide a way to screen entire orchards, tree by tree. The first method brings the laboratory to the field: a mobile GC-DMS (gas chromatograph-differential mobility spectrometer) unit is parked next to a tree (Fig. 1). The unit collects tree odors for 3 minutes. Ten minutes later, the GC-DMS unit provides a picture of the volatile profile, which is used to diagnose HLB in that tree.
as developed two volatile-based methods to detect HLB in citrus trees. Both methods provide a way to screen entire orchards, tree by tree. The first method brings the laboratory to the field: a mobile GC-DMS (gas chromatograph-differential mobility spectrometer) unit is parked next to a tree (Fig. 1). The unit collects tree odors for 3 minutes. Ten minutes later, the GC-DMS unit provides a picture of the volatile profile, which is used to diagnose HLB in that tree.For the second method (Fig. 2), samples
are collected in the field and returned to the laboratory. A Tic-Tac sized device called a Twister® is hung in the tree canopy for 2 hours. Twisters® are like odor sponges and absorb the chemicals released by the tree. Afterwards, the Twisters™ are collected and placed into vials, returned to the laboratory and tested
the field and returned to the laboratory. A Tic-Tac sized device called a Twister® is hung in the tree canopy for 2 hours. Twisters® are like odor sponges and absorb the chemicals released by the tree. Afterwards, the Twisters™ are collected and placed into vials, returned to the laboratory and tested
using a GC-MS (gas chromatograph-mass spectrometer), which provides the volatile profile.Once the odor profile is outlined by either GC-DMS or GC-MS, the Davis Lab uses machine learning algorithms that were developed over many years to describe the tree’s odor profile and determine if HLB is present.
The volatile profiling methods have been successful in detecting HLB infected trees and also differentiating trees that have citrus tristeza virus from those infected citrus stubborn (another bacterial disease) or HLB.
Who is working on the Project?
Dr. Cristina Davis, chair and professor in Mechanical & Aerospace Engineering at UC Davis, and her laboratory, led by Mitchell McCartney, are developing this method of early detection of HLB- infected trees.
What are the challenges and opportunities?
Dr. Davis has spun off a company from this research. XTB Laboratories is currently raising capital from investors to begin offering a commercial test. Visit xtblabs.com or more information.
As for potential opportunities, these technologies are also being used to determine whether new treatments are successful in either curing or preventing HLB infections.
Funding source: This project is funded by the U.S. Department of Agriculture, National Science Foundation, Citrus Research Board, and the Citrus Research and Development Foundation.
-
Canines can detect trees infected with the bacterium that causes huanglongbing
Research by Dr. Tim Gottwald
Article written by Tim Gottwald, Holly Deniston-Sheets and Beth Grafton-Cardwell.
Revised July 8, 2025.What is the technique?
Canines have a highly sensitive scent detection capability that is significantly better (parts per trillion) than most laboratory instruments and they can be trained to “alert” (either sit or lay) when they detect specific ‘smells’ (known as scent signatures). Most people are familiar with their ability to detect bombs, drugs, and plant material at airports. However, canines are also used to detect human pests, such as bed bugs, and agricultural pests, such as stink bugs, date palm weevils and imported fire ants. With regard to agricultural pathogens, canines have been shown to detect with greater than 98% accuracy the fungal pathogen that causes laurel wilt disease in avocado, the bacterium that causes citrus canker disease in citrus, and plum pox virus in peach orchards.
Researchers have been training and evaluating the efficacy of canines for detecting “Candidatus Liberibacter asiaticus” (CLas), the bacterium that causes huanglongbing (HLB), for 5 years in Florida, and CLas detection efforts with canines have recently begun in California. Dogs have been trained in both the laboratory environment and in the field. Researchers have demonstrated that well-trained canines can detect CLas over 95% of the time in commercial trees and over 92% of the time in residential trees. Researchers did not observe any differences in canine performance between citrus species and varieties. The training that the canines receive is very specific to CLas. When they are taken into citrus orchards infected with citrus tristeza virus, viroids, the fungal pathogen Phytophthora, or the bacterium that causes citrus stubborn, the CLas-trained canines do not respond to these diseases.
 The canines provide a significant opportunity to be used as an Early Detection Technology (EDT) in California. In a field study using potted citrus in Florida, dogs could detect CLas in some of the trees as early as 2 weeks after CLas-infected psyllids fed on the trees. In contrast, it can take 1-2 years for CLas to distribute itself in a mature citrus tree sufficiently for the bacterium to be present in sampled leaves, which are then tested and shown to be infected using laboratory techniques, such as Polymerase Chain Reaction (PCR). Using canines to detect early infections could significantly help reduce disease spread in California, where HLB is currently limited to southern areas of the state and identify areas where increased psyllid control measures are needed
The canines provide a significant opportunity to be used as an Early Detection Technology (EDT) in California. In a field study using potted citrus in Florida, dogs could detect CLas in some of the trees as early as 2 weeks after CLas-infected psyllids fed on the trees. In contrast, it can take 1-2 years for CLas to distribute itself in a mature citrus tree sufficiently for the bacterium to be present in sampled leaves, which are then tested and shown to be infected using laboratory techniques, such as Polymerase Chain Reaction (PCR). Using canines to detect early infections could significantly help reduce disease spread in California, where HLB is currently limited to southern areas of the state and identify areas where increased psyllid control measures are neededWho is working on the project?
Dr. Tim Gottwald, Research Leader and Epidemiologist at the USDA, U.S. Horticultural Research Laboratory in Fort Pierce, Florida, and additional collaborators with F1K9, LLC, USDA, North Carolina State University, Texas A&M University and the California Department of Food and Agriculture.
What are the challenges and opportunities?
The volatile scent signature associated with CLas-infection settles from the canopy and simultaneously emanates from root infections pooling at the base of the tree. The detector dog interrogates the tree holistically by alerting in seconds on the scent signature regardless of its origin (i.e., a single leaf, root, stem or the entire tree if systemically infected). Conversely, other detection technologies, like PCR, are reliant on selecting and processing a small amount of tissue from large trees and often miss incipient infections because infected tissue is so rare in newly infected trees. Early detection via dogs is devoid of these sampling issues. Therefore, it is difficult to confirm CLas detections by dogs using currently available molecular or chemical detection methods. Dogs have been tested in hot and cold temperatures and with wind speeds up to 20 MPH with no perceptible degradation in detection.
Human scouts require several minutes per tree to visually examine it for symptoms, then they must collect tissue which must be transported to a diagnostic lab for processing and analysis, which is time consuming and labor-intensive. Whereas, in a residential environment dogs can assess all trees in even large yards in a couple of minutes. The major limitation to the number of trees a dog can assess per day is access to these residential properties and the time required to relocate from property to property. In commercial groves a team of two dogs and one handler can survey a 10 acre planting (~1500 trees) in 1-2 hours depending on the number of infected trees; each positive alert requires rewarding the dog and tagging the infected tree. Dogs usually work 30 min then rest 30 min and can work 6-8 hours a day.
Utilizing dogs, CLas can be detected early in a region, when it is in just a few trees. If these few early infected trees are removed, the establishment and spread of the disease could be greatly reduced.
Like every detection instrument, dogs need to be periodically recalibrated. This is done by resensitizing them to known CLas-positive trees or specially prepared ‘scent pads’ that contain the scent signature of CLas to ensure they maintain > 98% accuracy of detection before being redeployed.
Gottwald, T. et al., 2020. Canine olfactory detection of a vectored phytobacterial pathogen, Liberibacter asiaticus, and integration with disease control. Proceedings of the National Academy of Sciences Feb 2020, 201914296; DOI: 10.1073/pnas.1914296117. https://doi.org/10.1073/pnas.1914296117.
Funding source: This project is funded by the USDA Farm Bill, USDA HLB Multiagency Committee (MAC), and USDA ARS program funds.
-
Starch accumulation sensor for early detection of HLB
Research by Dr. Alireza Pourreza, Department of Biological and Agricultural Engineering, University of California, Davis
Article written by Alireza Pourreza, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 9, 2025.What is the technique?

This technique uses starch accumulation in CLas bacteria-infected leaves as an early indication of huanglongbing (HLB) disease in citrus. Starch rotates the polarization plane of light. The sensor has a monochrome camera with a linear polarizing filter set perpendicularly to the polarizing film of the illumination system, which can then be used to visually detect patterns of starch deposition.

Starch accumulation in an HLB-infected leaf generates a blotchy mottle,
asymmetrical yellowing pattern. Deficiency of nutrients, like Mg and Zn,
cause more uniform yellowing symptoms in the leaf.The sensor, mounted on a gator vehicle, was tested in a Florida citrus grove. Polarized images were taken from healthy, HLB-infected, and Zn-
 deficient canopies. The sensor could detect and differentiate between HLB-infected and nutrient deficient plants. The polarized imaging methodology was also studied to determine the earliest time after infection HLB can be detected. In one study, two-year old Valencia orange plants were inoculated using a disk of infected leaf tissue grafted into a healthy leaf.
deficient canopies. The sensor could detect and differentiate between HLB-infected and nutrient deficient plants. The polarized imaging methodology was also studied to determine the earliest time after infection HLB can be detected. In one study, two-year old Valencia orange plants were inoculated using a disk of infected leaf tissue grafted into a healthy leaf.
Time-lapse polarized images of leaves from inoculated citrus plants were taken weekly. HLB symptoms (viewed as starch accumulation) became visible in the polarized images five weeks after inoculation, while plants were still not showing symptoms.
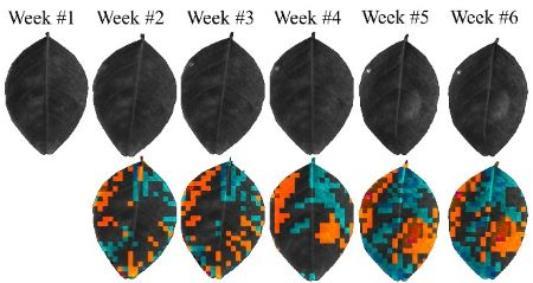
Who is working on the Project?
Alireza Pourreza, Department of Biological and Agricultural Engineering at the University of California, Davis, is part of a team of researchers (Won Suk Lee, John Schueller, Reza Ehsani, Ed Etxeberria, Bill Gurley, and Arunava Banerjee at the University of Florida and Eran Raveh at ARO Gilat Research Center, Negev, Israel) that developed and are testing the sensing system to measure starch accumulation in leaves resulting from infection by the CLas, bacterium, that causes HLB in citrus.
What are the challenges and opportunities?
Currently, the team is focusing on improving accuracy, determining how early in the infection this sensor can detect the disease, and developing a commercialized product for in-field diagnosis. This affordable tool will help citrus growers determine which trees are infected withCLas and, with tree removal, help growers to more effectively eliminate HLB from their groves in the early stages of infection.
Funding source: This project is funded by the Citrus Research and Development Foundation (CRDF)
-
Changes in microbial communities on citrus leaves can help detect HLB
Research by Dr. Johan Leveau, University of California, Davis
Article written by Johan Leveau, Brianna McGuire, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 9, 2025.What is the technique?
There are measurable differences between healthy citrus trees and citrus trees infected with the bacterium Candidatus Liberibacter asiaticus (CLas), that causes huanglongbing disease (HLB). The most obvious difference is the presence of CLas DNA in infected plants. Polymerase Chain Reaction (PCR) is a test that can detect CLas DNA and is currently the only way to confirm the presence of the bacterium in trees. However, PCR-based methods are costly and are typically used only to confirm CLas presence in suspect trees, rather than screen large numbers of trees for CLas infection. To reduce costs and so increase surveying, there is much interest in developing an alternative method of CLas detection, especially methods that take advantage of the tree-wide changes that occur upon infection. Research in the laboratory of Johan Leveau in the Department of Plant Pathology at UC Davis found that microbial communities living on the surface of citrus tree leaves change during CLas infection. Leveau’s team collects microbes from foliage by swabbing leaves, uses DNA-based methods to profile the bacteria and fungi that are present on the swab sample, and compares the profiles of CLas-infected and uninfected trees.
How can microbes be used to identify infected trees?
When a tree becomes infected with CLas bacteria, it responds by changing the chemical composition of its leaves, which in turn impacts the microorganisms that live on the leaf surface. Not all microorganisms are impacted equally, thus causing a shift in the microbial community: for some bacterial or fungal species the numbers go up, for others they go down. This shift can be measured after swabbing the leaves to collect the microbes, followed by extraction of bacterial and fungal DNA from the swab, and analyzing the DNA for the presence or absence of fungal or bacterial species. Some species are known to become more abundant after CLas infection, and these species, known as indicators, are the ones that may help determine whether a tree is infected with CLas and detect it early in its infection. This method is non-destructive to the tree and would allow for rapid processing once indicator microbes have been identified. Ideally, this method will function as a pre-diagnostic tool that helps prioritize trees for follow-up testing for CLas by PCR (Figure 1).
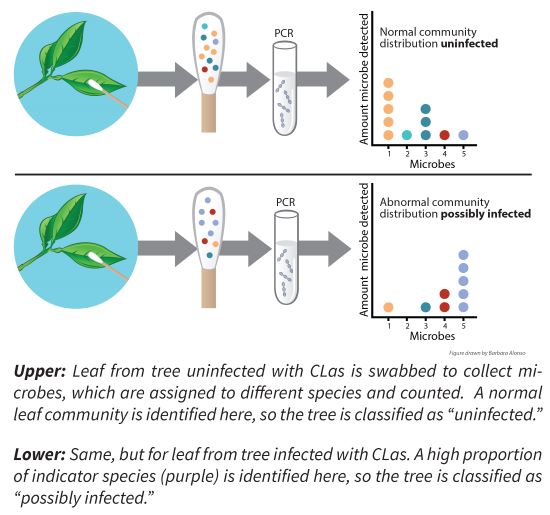
Who is working on the project?
Johan Leveau, is a Professor of Plant Pathology at the University of California, Davis. His research team uses these methods to identify and validate microbe indicators for early detection of HLB.
What are the challenges and opportunities?
The biggest challenge for this technique is finding microbial species that are true indicators of CLas infection, irrespective of the time of year, the region that the tree is grown in, the cultivar of the tree, the stage of infection of the tree, or the age of the tree. Therefore, microbes from a wide variety of trees and tree locations need to be analyzed. Many candidate taxa have already been identified as potential indicators of CLas infection but more testing is needed. Fortunately, more testing is on the immediate horizon. The Leveau lab’s early detection technology is currently being field-tested in several different screenings of healthy trees, trees infected with pathogens other than CLas, and trees infected with CLas. Combining the microbial swab test with other techniques, such as an antibody-based detection of indicator species, may increase the accuracy and volume of samples that can be used with this test.
Funding source: This project is funded by the Citrus Research Board.
-
Using antibodies for early detection of HLB infection
Research by Dr. Wembo Ma, University of California, Riverside
Article written by Wembo Ma, Sara Garcia Figuera, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 9, 2025.What is the technique?
Huanglongbing (HLB) is a disease caused by a bacterium called Candidatus Liberibacter asiaticus (CLas) that colonizes the phloem (nutrient conducting pathway) of citrus trees. To limit the spread of the disease, it is crucial to identify and remove infected trees as early in the infection process as possible. However, determining whether a tree is infected or not is very challenging. Visual inspection is not a suitable option because by the time symptoms appear (9 months or more), the Asian citrus psyllid insect vector has already moved the disease on to the next tree. The official biochemical method to detect the disease uses polymerase chain reaction (PCR). However, the PCR method requires that the leaf sample taken from the tree contains the bacterium and the bacterium might not always be in the leaf chosen, as CLas is often unevenly distributed in infected trees. Another approach could be to identify and detect molecules that the bacterium releases into the phloem early in the infection process. These molecules, which are smaller than the bacterium, would spread throughout the tree with the phloem flow, where they could be detected using an immunological method. Wenbo Ma and her collaborators have exploited this approach using released bacterial molecules called ‘effectors’ and a detection method called enzyme-linked immunosorbent assay (ELISA).
What are bacterial effectors?
Effectors are protein molecules secreted by pathogens such as bacteria to help promote disease in the plants that they infect. Effectors bind to other proteins and manipulate plant cell activity. Early in the infection process, as CLas colonizes the phloem of citrus plants, it secretes effectors into the phloem sap which then move throughout the plant in the vascular conducting tissue. Therefore, the effector molecules could be detected in areas of the plant where the bacterium is not present yet, before the plant shows any symptoms.
How can they be detected?
Bacterial effectors can be detected using antibodies, which are a type of protein from
the animal immune system that can identify specific protein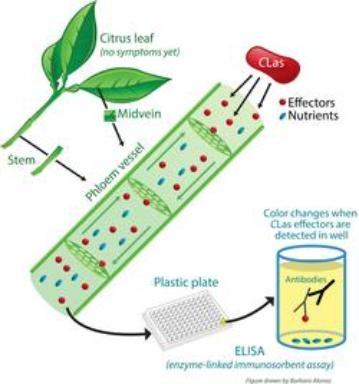 structures, like those in the effectors. By artificially exposing antibodies to the bacterial effectors, they can be ‘trained’ to detect them. ELISA (enzyme-linked immunosorbent assay) is a technique that combines these ‘trained’ antibodies with a color reaction, so that a visual signal is released if the antibodies detect the bacterial effector. A sample would be taken from the phloem tissue of a possibly infected tree and incubated with the antibodies in a small well of a plastic plate, in which the liquid would change color if the tree was infected with CLas. ELISA has been widely used for disease diagnosis because it is highly sensitive, rapid and inexpensive.
structures, like those in the effectors. By artificially exposing antibodies to the bacterial effectors, they can be ‘trained’ to detect them. ELISA (enzyme-linked immunosorbent assay) is a technique that combines these ‘trained’ antibodies with a color reaction, so that a visual signal is released if the antibodies detect the bacterial effector. A sample would be taken from the phloem tissue of a possibly infected tree and incubated with the antibodies in a small well of a plastic plate, in which the liquid would change color if the tree was infected with CLas. ELISA has been widely used for disease diagnosis because it is highly sensitive, rapid and inexpensive.Who is working on the project?
Wenbo Ma from the University of California, Riverside, has been leading this project with funding from the Citrus Research Board, USDA-NIFA and USDA-APHIS and the Office of Research, UC Riverside.
What are the challenges and opportunities?
Since effectors are essential for pathogenic bacteria to cause disease in plants, they should be present and detectable early in the infection process, before the plant shows any symptoms. And because they move systemically through the phloem, they could be detected in areas of the plant that the bacterium has not yet colonized, overcoming the problem of uneven distribution inherent to the PCR-based detection method. The team led by Wenbo Ma is working to determine how early in the infection process this technique would detect the bacterial effectors, validating it with different citrus varieties, tree ages and sampling protocols. Another challenge is the expense and time requirements of ELISA, which limits how fast samples could be processed. It would be difficult to use it as a rapid screen for entire citrus orchards. Further experiments will need to be conducted before this early detection technique is deployed for field use, but it could be a valuable complement to other HLB diagnostic tools.
Funding source: This project is funded by the Citrus Research Board, USDA-NIFA and USDA-APHIS and the Office of Research, UC Riverside.
-
The value of earl detection technologies (EDTs) for HLB management
Research by Dr. Neil McRoberts, University of California, Davis
Article written by Neil McRoberts, Sara Garcia Figuera, Jennifer Reed and Brianna McGuire.
Revised June 25/2025What is the technique?
Early detection technologies (EDTs) are tests that indicate the presence of disease before signs or symptoms of the disease can be seen. In the same way that a doctor measures a patient’s blood pressure to look for heart problems, a grower might use a trained “sniffer” dog to detect changes in a tree that looks healthy but has huanglongbing (HLB) disease. By using the EDT, the grower is able to uncover HLB earlier, and can decide on an early, cost-saving course of action.
In the case of HLB, there are many EDTs under development. Some of them look for patterns in the microorganisms that live on the citrus leaves (Leveau snapshot); some look for patterns in the chemicals that are produced by the tree in response to HLB (Pourreza, Davis and Slupsky); and others look for the molecules that the bacterium injects in the tree to cause disease (Ma).
Why do we need EDTs for HLB?
To understand why EDTs are needed and what their potential value is, it is necessary to understand the difference between the incubation period for a disease and the latent period. The incubation period is the time between exposure to the pathogen and the appearance of symptoms. The latent period is the time between exposure and the newly-infected host becoming infectious. Huanglongbing (HLB) has a long incubation period and a very short latent period, which means that a tree can be diseased for a long time without showing any visible symptoms, while being infectious for a large fraction of that time. Even if a tree does not seem diseased, it can serve as a home for the bacterium (Candidatus Liberibacter asiaticus, CLas) that causes HLB. If a psyllid feeds on the infected tissue of a tree (with or without symptoms), CLas that is present in the leaf tissue can be picked up by the insect and transmitted to other trees when the psyllid moves on to feed. Information from an EDT can help a grower detect the disease in a tree a long time before it would be detected by eye. This cuts down the time psyllids are able to feed on it and transmit the disease, slowing the spread of HLB to neighboring trees.
Why is it important to remove infected trees as early as possible?
If a tree that tests positive for CLas is not treated or removed, the bacterium will spread throughout the tree. Over time, an increasing proportion of the tree’s tissues will become infected, increasing the chances that a psyllid will become infected upon feeding, and subsequently spread the infection to healthy neighboring trees. If the infected tree is removed, there is no opportunity for psyllids to feed on the infected tissue and spread the disease. Once CLas is detected, tree removal is the only surefire way to prevent the spread of the infection, and it is extremely time-sensitive. The sooner an infected tree is removed, the lower the chances that psyllids will get infected. The savings associated with early infected tree removal will be proportional to the amount of surrounding trees that would have been infected with CLas due to that tree, and the number of months that it would be left on the ground.
Who is working on this project?
Several research teams in different universities and research stations, supported by a variety of funding organizations, have been working on the development of a variety of EDTs. These EDTs, designed under laboratory and greenhouse conditions, are being validated under field conditions in Texas and Florida. In California, where HLB has not been detected in citrus orchards, samples of different citrus varieties have been collected from healthy trees and trees affected by other diseases from all over the state. These samples are being used to calibrate the EDTs, and to test if they can distinguish between healthy and HLB-diseased trees, and between HLB-diseased trees and trees affected by other common citrus diseases. Dr. Neil McRoberts and his team at UC Davis are evaluating the data from these experiments and providing support to the EDT researchers.
What are the challenges and opportunities?
Currently, regulations require HLB infected trees to be removed if a certain amount of CLas DNA is detected in leaf samples through polymerase chain reaction (PCR). However, CLas is unevenly distributed in the sap of citrus trees, and the leaf samples collected might not be PCR-positive even though the bacterium is already present elsewhere in the tree. EDTs offer the possibility to detect infected trees before they are PCR-positive, so they could be removed earlier in the HLB epidemic. Therefore, the value of EDTs relies on the voluntary removal of EDT-positive trees before the law requires them to be removed.
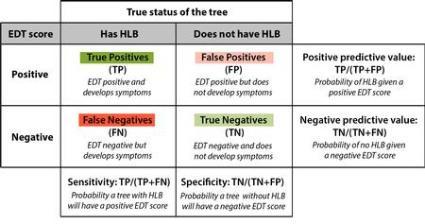
No EDT gives perfect diagnostic results. Sometimes healthy trees will produce EDT scores that look like diseased trees (so-called “false positives” - see Table 1). Removing such trees will result in an immediate financial loss. However, because the economic damage caused by leaving an infected tree in place is much bigger than the value of a healthy tree, using an EDT to guide decisions has the potential to result in a long-term economic benefit to individual growers and communities, by reducing the spread of HLB. Losing a few healthy trees along the way is the unavoidable cost of stopping the disease from spreading. Likewise, some trees will seem healthy based on EDT scores but might end up showing symptoms (“false negatives”- see Table 1). The proportion of true positives, false positives, true negatives and false negatives represents the accuracy of a diagnostic test. Dr. McRoberts’ team is analyzing the accuracy of the EDTs, and preliminary results suggests the best performing EDTs could be correctly determining the status of the trees 95% of the time.
The results of this analysis could be used to foster the adoption of EDTs among the citrus grower community, promoting the idea that the sooner infected trees are detected and removed, the smaller impact HLB will have on California’s citrus production. Unless there is sufficient cooperation in integrated management of HLB by removing infected trees as early as possible, controlling the ACP on an area-wide scale, and using certified plant material, the California citrus industry is likely to suffer unsustainable economic losses to HLB.
Funding source: This project is funded by the Citrus Research Board and the HLB Multi-Agency Coordination Group.