General Topics
The snapshots on this page provide background information on genetic engineering and editing approaches for plant modification and HLB detection.
-
New genome editing technologies - CRISPR
Research by Dr. Peggy Lemaux, UC Berkeley, California
Written by Drs. Peggy Lemaux, Becky Mackelprang (UC Berkeley) and Elizabeth Grafton-Cardwell (UC Riverside).
Revised June 24, 2025.What are older genetic modification techniques?
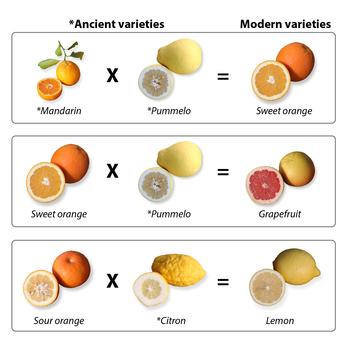 Our ancestors had no idea that the genetic information in a cell, its DNA, is passed down from one generation to the next, and is responsible for the way plants look, taste, and how much food they produce. But they figured out that through a process, called selective breeding, they could breed two plants with different, valuable traits, like high yield and disease resistance, to create offspring with both of these valued traits. Over time, changes from the breeding process resulted in dramatic changes in the plant’s DNA and consequently, in the way the citrus family looks and tastes. For example, mandarin and pummelo crosses created the sweet oranges of today (Figure 1). This approach resulted in citrus varieties with genetic modifications, but they are not what the popular press today term “genetically modified organisms” or GMOs.
Our ancestors had no idea that the genetic information in a cell, its DNA, is passed down from one generation to the next, and is responsible for the way plants look, taste, and how much food they produce. But they figured out that through a process, called selective breeding, they could breed two plants with different, valuable traits, like high yield and disease resistance, to create offspring with both of these valued traits. Over time, changes from the breeding process resulted in dramatic changes in the plant’s DNA and consequently, in the way the citrus family looks and tastes. For example, mandarin and pummelo crosses created the sweet oranges of today (Figure 1). This approach resulted in citrus varieties with genetic modifications, but they are not what the popular press today term “genetically modified organisms” or GMOs.Engineering
A modern method of modifying the genetics of a plant involves cutting out DNA that codes for a particular trait, like that responsible for the sweetness of a mango, and introducing it into another plant, such as a lemon, to make it sweeter. This is possible because the “language” of traits—the DNA—is the same for citrus and mango (and for all life on our planet). For more information on these engineering technologies, see the “Genes, Genomes and Genetic Engineering in Citrus” Snapshot. Today, there are commercially available genetically engineered (GE, GMO) varieties of alfalfa, canola, corn, cotton, soy and sugar beet, as well as smaller acreages of papaya, summer squash, sweet corn, apples and potatoes. These crop varieties have had DNA from another plant or organism cut and pasted into their own DNA. Currently there are no GE/GMO citrus varieties in the market.
Editing
When engineering or pasting (new genetic information into a plant) researchers are unable to choose where in all of the genetic information in the cell (the genome) the new piece is inserted. Additionally, making an engineered variety is a slow and expensive process, potentially costing $10-$20M for each new engineered variety.
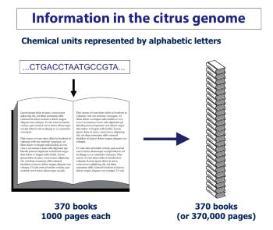
Recently, researchers developed other tools to genetically alter plants that are more precise, more economical, and faster. Numerous so-called gene-editing techniques were created starting in the ‘90s. The most widely used today – the CRISPR/Cas systems – allow tailored modifications at specific locations in the plant genome. This gene-editing technology relies on very precisely inserting or editing information at a targeted spot in the 370,000 pages of information in the citrus genome (Figure 2).

But, making these changes is done in a more directed way, and they can result in either mutations or modifications at precise, selected locations in the genome. When gene-editing is used to make a mutation, a few letters of DNA are 1) exchanged for others, 2) deleted, or 3) inserted. In the first case, a new word with a different meaning is made – for example “includes” becomes “excludes” (Figure 3).
In this case the original and edited words have the same number of letters but the meaning changes. When deletion mutations occur, letters are removed so “includes” becomes “in.” In a sentence, changing “include” to “in” can drastically change the sentence’s meaning. So, too, can deleting a few letters in a DNA sequence change the meaning or function of that sequence. Finally, adding letters to a sentence or a segment of DNA can change its meaning. For example, adding “un” to make “unexcluded” changes the word’s meaning. Mutations like these can also occur naturally or can be induced by human intervention. The resulting varieties can be used in conventional and organic farming.

Gene-editing can also be used to make plant modifications. In the genetic analogy, we can add information. In this case, “citrus includes members like lemons and limes” becomes “citrus includes members like lemons and limes plus more diverse varieties like mandarins and kumquats” (Figure 4).
In the CRISPR-Cas9 gene-editing system, Cas9 is a protein that acts as a molecular scalpel to cut DNA very selectively and very efficiently at a particular location, a CRISPR site, where it is programmed to make a change. This causes a mutation, like those described above, or an edit to be made that changes the trait in a desired way. This technology has already been used in some crops to reduce a plant’s disease susceptibility, to knock out genetic information for allergens, or to stop mushrooms from browning.
Who is working on the Project?
To date some gene editing efforts have been initiated in citrus and in the psyllid, although none have yet entered the commercial marketplace as an improved product. For the psyllid, the goal would be to develop a psyllid that cannot transmit the disease and then flood the natural populations with this new insect variety. For the citrus tree, the goal would be to develop a tree that can withstand HLB disease and still maintain productivity.
What are the challenges and opportunities for editing?
While there are large acreages of engineered (GMO) crops being grown commercially, particularly in the U.S. and South America, the numbers of crops and traits are limited almost exclusively to insect tolerance and herbicide tolerance. Only a few examples of other traits have emerged commercially – disease resistance in summer squash, papaya and potato and delayed browning in apples and potato. Release of engineered varieties has been slow due to high regulatory costs, intellectual property hurdles and consumer acceptance issues.
Currently there are no edited crop or psyllid varieties being grown commercially. There is, however, great potential for editing technology to knock out traits that are, for example, needed for the Clas bacterium to propagate in citrus cells or for the psyllid to support the replication of the bacterium in its cells. The key challenge remains as to whether consumers would accept edited citrus, should a successful approach be demonstrated.
-
Genes, Genomes and Genetic Engineering in Citrus
Research by Dr. Peggy Lemaux, UC Berkeley, California.
Written by Drs. Peggy Lemaux and Elizabeth Grafton-Cardwell.
Revised 6/24/2025.Living organisms, like plants, have many cells – each with genetic information to specify how plants look, taste and feel. This genetic information also determines whether plants can be infected by disease-causing organisms, like the bacterium causing Huanglongbing (HLB) and the degree of damage caused by the disease.
That genetic information, containing long strings of chemicals called DNA, is organized in individual units, called genes, that specify traits, like a lemon’s tartness or a tangerine’s color. All of the genetic information, called a genome, is like a collection of books with information on many topics. Each organism has its own set of books – with some of the same information and some different - making lemons look and taste different from oranges and limes.
If alphabetic letters are used to represent each chemical in the long DNA string, it would require about 35 books, each of 1000 pages, to contain all information in a citrus cell. All of the chemical units in sweet orange are known, revealing nearly 25,000 genes.
How is classical breeding used to make new citrus varieties?
Most plants develop roots, stems and leaves after seeds germinate. But mature trees of citrus, and other tree crops, grow from two parts of other trees, grafted together. The rootstock is mostly below ground. The scion forms the tree’s upper part, its trunk, branches, leaves and, most importantly, fruit.
One way breeders create new citrus varieties is to select rootstocks and scions that provide different improvements. In rootstocks, they look for traits for tree size, yield, disease and insect tolerance, and scion compatibility. In the scion, they look for fruit size and flavor, rind and flesh color, ratios of acid and sugar and the numbers of seeds.
To create a new rootstock, or scion, breeders cross two citrus varieties. In crosses, pollen (male, sperm cells) is taken from one tree and delivered to the egg (female cell) of another tree. Male and female cells fuse, divide and become seeds for the next generation, which has new mixes of genes and traits. Many common citrus types, like oranges, lemons, and limes, originated long ago from such crosses of citrons, mandarins and other early varieties. For example, the sweet orange came from a cross between a mandarin and a pummelo. What happens to the 35 books of genetic information from each parent? Genetic rules dictate the resulting tree can only have 35 books, half randomly received from one parent, half from the other, resulting in next generation trees with unique combinations of genes and traits.

Other methods of creating new citrus varieties
Besides classical breeding, the order or nature of the chemical units can be changed through the effects of sunlight on DNA. This action results in mutations, or genetic changes. The pink-fleshed, navel orange, Cara Cara, arose from a mutation in a Washington navel orange tree. Irradiating citrus budwood can also act like sunlight and rapidly induce genetic changes, an approach that scientists used to create the low-seeded mandarins Tango, Daisy SL, Fairchild LS and Kinnow LS.
More recently breeders have used marker-assisted selection (MAS), which involves creating a “table of contents” with tags for locations of genes specifying certain traits. Breeders use the naturally occurring DNA tags to identify whether desired genes are present. In citrus, MAS has been used on a very limited basis in, for example, rootstock breeding programs to introduce citrus nematode resistance.
What is a genetically engineered (GE) organism or GMO?
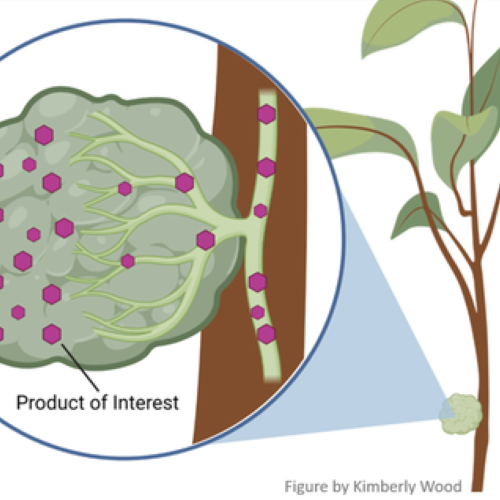
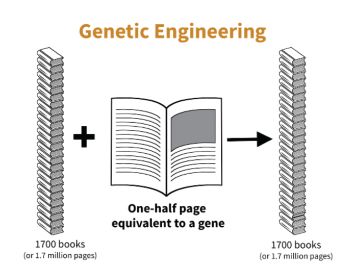 DNA in all organisms is written in the same chemical language, so genetic information can be exchanged among organisms. How could this be used to improve citrus? Once genetic information for useful traits is identified, it can be introduced into genetic information in the citrus cell, using a cut and paste process, like that in a word processing system. Once the trait’s chemical text is found (equal to about a half-page of information), chemical scissors are used to remove the information, and chemical paste is used to place that information stably into the DNA of the same or another plant. This process, termed genetic engineering (GE), gives rise to plants with modified genetic information – often termed GMO (genetically modified organism).
DNA in all organisms is written in the same chemical language, so genetic information can be exchanged among organisms. How could this be used to improve citrus? Once genetic information for useful traits is identified, it can be introduced into genetic information in the citrus cell, using a cut and paste process, like that in a word processing system. Once the trait’s chemical text is found (equal to about a half-page of information), chemical scissors are used to remove the information, and chemical paste is used to place that information stably into the DNA of the same or another plant. This process, termed genetic engineering (GE), gives rise to plants with modified genetic information – often termed GMO (genetically modified organism).How can breeding and engineering methods be used to protect against HLB?
Citrus fruits and their relatives originated in South-east Asia, New Caledonia and Australia and they have been cultivated in tropical and temperate parts of the world for more than 2000 years. During that time, natural crossing and mutations have occurred but, since the late 1980’s, breeders have made intentional crosses and identified natural mutations, like the pink flesh of the Cara Cara orange. But fruit tree breeding is slow, taking 10-15 years to identify parents with desirable traits, crossing them and then waiting for fruit. After that, a few more years to insure there are no unwanted characteristics and trees and fruits thrive. In order to use conventional breeding to combat HLB, there must be a source of resistance within a sexually compatible variety and to date this has not been identified within citrus.
In citrus, genetic engineering has been used in experimental approaches to protect against diseases, to provide certain insect resistance, to create dwarf varieties and to afford drought and salt tolerance. In more recent experiments, scientists are now using genes from other citrus varieties to engineer citrus with lower acidity, unique blood orange colors, increased disease resistance and lower levels in grapefruit of the chemical that interferes with statin drugs. Further along, but still experimental, with regard to HLB, a population of genetically engineered sweet orange trees, with a piece of genetic information from watercress, was created in 2017 and is currently being monitored for HLB resistance. Several lines have remained HLB-negative for over five years at a site that has >95% HLB infection. There are other genes, like those from spinach, that are currently being assessed for protection against HLB, thus demonstrating potential benefits from genetic engineering. A new genetic tool, termed genome editing, is being explored to identify new approaches for resistance within the citrus family.
What are the challenges for breeders?
The challenges for breeders using conventional approaches are finding a citrus relative that has resistance to HLB and the long amount of time it takes to do crosses and determine if the approach has created a resistant variety. In the case of genetic engineering, researchers have to identify genes that are effective against the bacterium causing HLB and insert them into the citrus plant. Then time is required to assure that the trees are protected against the bacterium and remain protected over the long-term. Finally, even if an effective approach is found with either classical breeding or genetic engineering, it has to be introduced into all varieties of interest and then orchards have to be replanted.
-
How is the HLB-associated bacterium detected in citrus trees and Asian Citrus Psyllids?
Research by Dr. Greg McCollum, USDA, ARS, Fort Pierce, FL.
Article written by Greg McCollum, Brianna McGuire, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 24, 2018.How can detection of CLas infections impact the development of HLB epidemics?
Candidatus Liberibacter asiaticus (CLas) is considered to be the bacterial pathogen that causes the incurable disease Huanglongbing (HLB) in citrus. CLas is transmitted to citrus trees when the insect vector, Asian citrus psyllid (ACP), feeds on plant tissue or when infected plant tissue is grafted onto a citrus tree. No matter how CLas arrives, when ACP invades citrus-growing regions, CLas bacterial infection and HLB soon follow. Maintaining ACP populations as low as possible by aggressive insecticide applications has been clearly shown to slow the spread of HLB. However, ACP can never be 100% eliminated by insecticides. So, if there are CLas-infected trees and even small populations of ACP, the incidence of HLB will increase over time. If trees infected with CLas can be identified and removed as early as possible, the chance of slowing an HLB epidemic improves significantly.
What is the problem?
Detection of CLas in citrus and ACP is necessary for regulatory action (tree removal) and to guide psyllid and disease management strategies. In general, confirming a pathogen’s presence in a host is usually accomplished by isolating the pathogen from tissue suspected to be infected, growing the pathogen in a sterile environment to obtain a pure culture, followed by several means of identification (examining the appearance of cultured cells, determining a pathogen’s DNA sequence, etc.). Unfortunately, culturing CLas outside of either citrus or ACP has not yet been possible, making confirmation of infection especially challenging. At present, the only means to confirm CLas infection is by detection of CLas DNA in extracts prepared from infected citrus tissue or psyllids.
What is the technique?
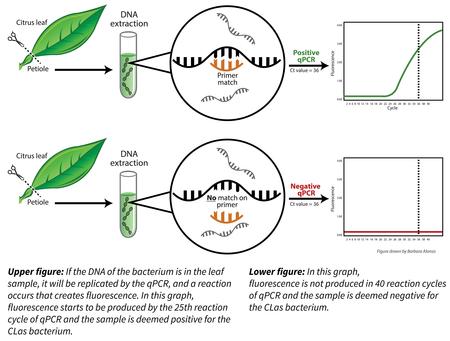
For citrus extracts and psyllids, the most sensitive and specific technology for detection of CLas DNA is the polymerase chain reaction (PCR) test. DNA is the molecule that contains the genetic information for all life, found in the cells of all organisms. PCR is a technique used to rapidly copy a very small section of DNA sequence so that it can be detected. PCR is somewhat similar to a copy machine. For example, if a single piece of paper is placed in a copy machine and the copy button is pressed 36 times, the end result is 37 copies.
For PCR, starting with a single copy of the DNA target sequence and running the PCR for 36 cycles results each copy making copies of itself, resulting in about 7 billion copies of the original target sequence.
A version of PCR, known as quantitative PCR (qPCR), is the regulatory standard for diagnosing CLas. In qPCR, each copy of target DNA sequence generated gives off a fluorescent signal, and that signal is measured at the end of each PCR cycle. The results of qPCR assays are expressed as the number of PCR cycles required to produce a fluorescent signal of a specific intensity, which is an indication of the starting number of copies of the target DNA. This value is known as the crossing threshold (Ct). The regulatory threshold for a positive plant sample is a Ct of 36, which means that it takes 36 or fewer cycles for sufficient fluorescence to be detected. A plant sample with a Ct value lower than 36 is defined as positive for CLas. If the Ct value is > 37, then the sample is viewed as either noise in the system, a very weak positive, or a negative. The test’s ability to detect a true positive becomes less reliable if there are fewer than three CLas cells in a plant sample.
Plant samples are prepared for PCR by chopping up and freeze drying the petioles, the slender stalks that join leaves to stems, of many leaves on a tree that is suspected to have the disease. These freeze-dried petioles are then ground, and DNA is extracted. qPCR is performed on these DNA extracts. By California law, a tree with a positive qPCR reading must be destroyed.
Psyllid DNA is also tested for CLas presence with qPCR. Up to 25 live psyllids collected from a tree are preserved in alcohol, taken to the laboratory, and crushed to create one unified sample. In California, positive psyllids do not result in tree removal, because it is not necessarily known where the psyllids picked up the bacterium. However, psyllid testing is very important as an early warning system to find the region where the bacterium is present so leaf testing can be intensified.
Who is working on the Project?
Greg McCollum, Research Plant Physiologist, USDA, ARS, Fort Pierce, FL and his research team are working to determine how soon CLas infections can be detected with qPCR following ACP feeding on citrus as well as how CLas moves in citrus. They are also collaborating with researchers at UC Riverside and UC Davis to validate technologies to improve early detection of CLas infections.
What are the challenges and opportunities?
Although qPCR is a very sensitive and specific method for detection of CLas DNA, qPCR is severely limited by sampling and testing capacity. It is only possible to evaluate 100 mg of tissue (about equal to a grain of rice) per sample. Because CLas is typically not evenly distributed in the citrus canopy but found in individual leaves or isolated to a branch, finding an infected leaf is effectively random, unless HLB symptoms are visible. If there are 10,000 leaves on a tree and the CLas infection is limited to a single branch on the tree, and one leaf is collected, the chance of finding CLas is only 1 in 10,000. Though the odds of finding CLas increases as HLB symptoms progress beyond one branch, it is critical to find the infection while limited to one branch, before ACP spreads further. Other early detection technologies (EDTs) measure a whole-tree immune response by CLas-infected trees or rapid distribution of bacterial byproducts throughout the tree rather than the quantity of CLas in a sample of leaves. There are numerous EDTs being studied - please see other EDT snapshots on this website for more details. These approaches could be used to identify quiescent infections prior to qPCR diagnostics, which would result in more efficient and effective use of qPCR resources and would enable regulators to remove trees before psyllids further spread HLB.
Funding source: This project is funded by the USDA, ARS, USDA, APHIS, Citrus Research Board, and California Citrus Nursery Advisory Board.
-
The value of earl detection technologies (EDTs) for HLB management
Research by Dr. Neil McRoberts, University of California, Davis
Article written by Neil McRoberts, Sara Garcia Figuera, Jennifer Reed and Brianna McGuire.
Revised June 25/2025What is the technique?
Early detection technologies (EDTs) are tests that indicate the presence of disease before signs or symptoms of the disease can be seen. In the same way that a doctor measures a patient’s blood pressure to look for heart problems, a grower might use a trained “sniffer” dog to detect changes in a tree that looks healthy but has huanglongbing (HLB) disease. By using the EDT, the grower is able to uncover HLB earlier, and can decide on an early, cost-saving course of action.
In the case of HLB, there are many EDTs under development. Some of them look for patterns in the microorganisms that live on the citrus leaves (Leveau snapshot); some look for patterns in the chemicals that are produced by the tree in response to HLB (Pourreza, Davis and Slupsky); and others look for the molecules that the bacterium injects in the tree to cause disease (Ma).
Why do we need EDTs for HLB?
To understand why EDTs are needed and what their potential value is, it is necessary to understand the difference between the incubation period for a disease and the latent period. The incubation period is the time between exposure to the pathogen and the appearance of symptoms. The latent period is the time between exposure and the newly-infected host becoming infectious. Huanglongbing (HLB) has a long incubation period and a very short latent period, which means that a tree can be diseased for a long time without showing any visible symptoms, while being infectious for a large fraction of that time. Even if a tree does not seem diseased, it can serve as a home for the bacterium (Candidatus Liberibacter asiaticus, CLas) that causes HLB. If a psyllid feeds on the infected tissue of a tree (with or without symptoms), CLas that is present in the leaf tissue can be picked up by the insect and transmitted to other trees when the psyllid moves on to feed. Information from an EDT can help a grower detect the disease in a tree a long time before it would be detected by eye. This cuts down the time psyllids are able to feed on it and transmit the disease, slowing the spread of HLB to neighboring trees.
Why is it important to remove infected trees as early as possible?
If a tree that tests positive for CLas is not treated or removed, the bacterium will spread throughout the tree. Over time, an increasing proportion of the tree’s tissues will become infected, increasing the chances that a psyllid will become infected upon feeding, and subsequently spread the infection to healthy neighboring trees. If the infected tree is removed, there is no opportunity for psyllids to feed on the infected tissue and spread the disease. Once CLas is detected, tree removal is the only surefire way to prevent the spread of the infection, and it is extremely time-sensitive. The sooner an infected tree is removed, the lower the chances that psyllids will get infected. The savings associated with early infected tree removal will be proportional to the amount of surrounding trees that would have been infected with CLas due to that tree, and the number of months that it would be left on the ground.
Who is working on this project?
Several research teams in different universities and research stations, supported by a variety of funding organizations, have been working on the development of a variety of EDTs. These EDTs, designed under laboratory and greenhouse conditions, are being validated under field conditions in Texas and Florida. In California, where HLB has not been detected in citrus orchards, samples of different citrus varieties have been collected from healthy trees and trees affected by other diseases from all over the state. These samples are being used to calibrate the EDTs, and to test if they can distinguish between healthy and HLB-diseased trees, and between HLB-diseased trees and trees affected by other common citrus diseases. Dr. Neil McRoberts and his team at UC Davis are evaluating the data from these experiments and providing support to the EDT researchers.
What are the challenges and opportunities?
Currently, regulations require HLB infected trees to be removed if a certain amount of CLas DNA is detected in leaf samples through polymerase chain reaction (PCR). However, CLas is unevenly distributed in the sap of citrus trees, and the leaf samples collected might not be PCR-positive even though the bacterium is already present elsewhere in the tree. EDTs offer the possibility to detect infected trees before they are PCR-positive, so they could be removed earlier in the HLB epidemic. Therefore, the value of EDTs relies on the voluntary removal of EDT-positive trees before the law requires them to be removed.
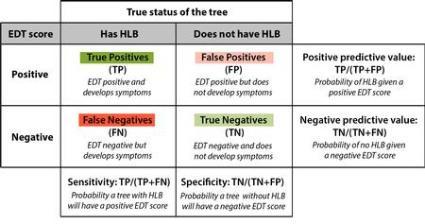
No EDT gives perfect diagnostic results. Sometimes healthy trees will produce EDT scores that look like diseased trees (so-called “false positives” - see Table 1). Removing such trees will result in an immediate financial loss. However, because the economic damage caused by leaving an infected tree in place is much bigger than the value of a healthy tree, using an EDT to guide decisions has the potential to result in a long-term economic benefit to individual growers and communities, by reducing the spread of HLB. Losing a few healthy trees along the way is the unavoidable cost of stopping the disease from spreading. Likewise, some trees will seem healthy based on EDT scores but might end up showing symptoms (“false negatives”- see Table 1). The proportion of true positives, false positives, true negatives and false negatives represents the accuracy of a diagnostic test. Dr. McRoberts’ team is analyzing the accuracy of the EDTs, and preliminary results suggests the best performing EDTs could be correctly determining the status of the trees 95% of the time.
The results of this analysis could be used to foster the adoption of EDTs among the citrus grower community, promoting the idea that the sooner infected trees are detected and removed, the smaller impact HLB will have on California’s citrus production. Unless there is sufficient cooperation in integrated management of HLB by removing infected trees as early as possible, controlling the ACP on an area-wide scale, and using certified plant material, the California citrus industry is likely to suffer unsustainable economic losses to HLB.
Funding source: This project is funded by the Citrus Research Board and the HLB Multi-Agency Coordination Group.