Ongoing NIFA Projects
The snapshots on this page describe methods progress in ongoing USDA NIFA-funded research to combat HLB.
-
Accelerating implementation of HLB-tolerant hybrids as new commercial cultivars for fresh and processed citrus
Research by USDA Agricultural Research Service in Ft. Pierce FL, University of Florida in Lake Alfred, and Ft. Pierce FL; and the University of California, Riverside CA.
Article written by Jinhe Bai, Anne Plotto, Ed Stover, Matt Mattia, Fred Gmitter and Mikeal Roose
Article edited by Peggy Lemaux, Lukasz Stelinski
Revised July 15, 2025.What is the research?
Breeding and selecting HLB-tolerant varieties will provide the most sustainable solution to this devastating disease. This project includes validation of HLB-tolerance in the field, identification of genetic markers associated with tolerance to expedite varietal screening, and characterization of the fruit and juice quality of the tolerant selections. Due to the urgent need of maintaining the Florida juice industry, a major focus has been to select fruit with sweet-orange-like traits for juice. Many thousands of trees have been evaluated in the field for these traits. Standards and trees showing some HLB-tolerance, from over fifty hybrids and selected commercial cultivars, were harvested over a course of five years for juice analysis. Fruit was evaluated for juice chemistry, as well as sensory traits using a trained tasting panel.
What are major successes to date?
Tasting panel tests confirmed the superior orange-like flavor of U.S. SunDragon and Sugar Belle®, and verified their suitability for blending with sweet-orange juice. Some named cultivars and selections from breeding programs also had good field performance and good quality but most were more like mandarin oranges, sweet and tasty, but flavor was not similar to sweet orange.
HLB-tolerance and good fruit traits have been shown in conventional citrus and also in hybrids derived from more distant citrus relatives. This greatly expands the diversity that can contribute to commercial citrus and strengthens the likelihood of durable HLB-tolerance.
Extensive data have been collected on fruit quality and HLB-tolerance. Tree genetics are being analyzed to provide genetic markers for the traits that will permit rapid screening in breeding populations. We anticipate that this work will provide HLB-tolerant selections for use in juice or as fresh fruit and will accelerate identification of the best material among 50,000 hybrids recently produced in citrus breeding programs. This work will also help identify volatiles and other chemicals that contribute most to orange or mandarin flavor.
Who is working on this project?
Bai, A. Plotto, R. Shatters, E. Stover, J. Manthey and M. Mattia from USDA Fort Pierce; F. Gmitter, Y. Wang, and M. Ritenour from U Florida; and M. Roose from U of California, Riverside.
What are the challenges and opportunities?
The team has completed four years of a five-year project and extensive data have been collected. However, identification of screening markers for HLB tolerance and juice quality is hampered by variability in effects caused by HLB between plants due to variable time of infection between trees for this slow-developing disease and likely variable root adaptation of trees grown from seed rather than produced by grafting. While genetic analysis is fast and relatively affordable, collection of tree and fruit data on thousands of trees remains expensive and time-consuming. Identification of accurate and high through-put assessments are underway and will be critical to ultimate success.
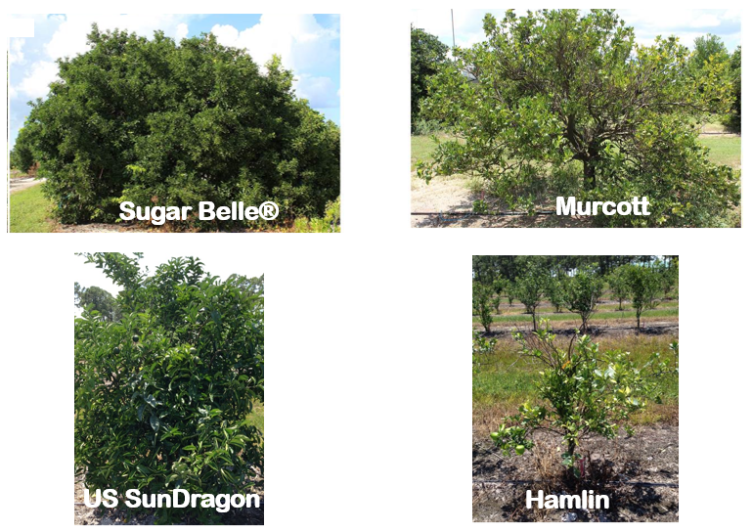
Figure 1: Upper section compares HLB-tolerant Sugar Belle to HLB-sensitive Murcott (photos F. Gmitter). Lower section compares HLB-tolerant US SunDragon to HLB-sensitive Hamlin (photos E. Stover). All trees are growing in typical HLB-affected Florida commercial groves.
Funding source: USDA-National Institute of Food and Agriculture (NIFA) - Citrus Disease Research and Extension Program # Grant 2018-70016-27453
-
Development of antimicrobial peptides from citrus to kill the CLas bacterium causing HLB
Research by: USDA, Horticultural Research Lab, Ft. Pierce and New Mexico Consortium
Article written by: Ed Stover, Goutam Gupta
Article edited by: Lukasz Stelinski, Peggy G. Lemaux
Revised July 14, 2025.What is the technique?
Antimicrobial peptides (AMPs) are small proteins produced by plants and animals as a first line of protection against pathogens by disrupting the pathogen’s cells. Our overall goal is to suppress CLas and maintain health in HLB-affected citrus. All organisms produce many peptides (small proteins), which serve many functions, including some unrelated to disease resistance. In this research, we are focusing on a specific subset of AMPs, derived from citrus genes, that will be used to kill CLas that causes HLB disease.
How are these AMPs developed and tested?
These citrus-derived AMPs have shown excellent activity against CLas in the lab and greenhouse. The goal of this NIFA project is to evaluate their efficacy as killing agents against CLas and therapeutics for existing infected trees to sustain commercial citrus production. In addition, we are developing trees that themselves express these AMPs to provide a form of HLB resistance.
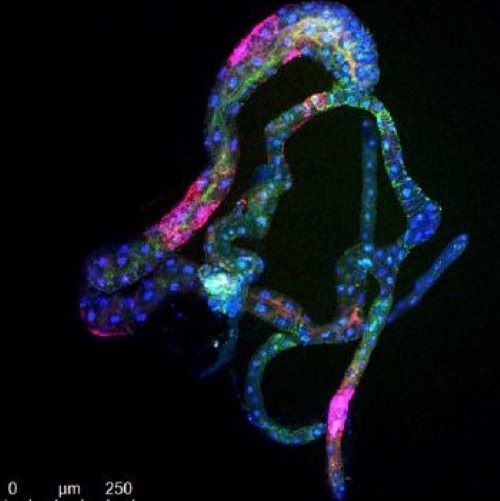
The latest versions of the anti-HLB AMPs consist of two different types of citrus peptides linked together (see Figure). After scouring the citrus gene sequence data, candidate peptides are selected which are first tested for interactions with CLas membranes using computer simulation. The best AMPs from these studies are then tested in lab cultures for their ability to kill bacteria relate
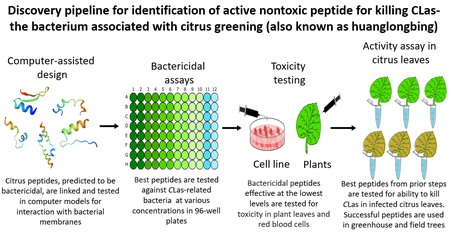 d to CLas. The promising candidate AMPs are then tested for toxicity to plants and animal cells. AMPs that pass these tests are then tested in HLB-affected citrus trees in the greenhouse and then in the field, to determine if they destroy CLas and enhance tree health. This process is outlined (Figure 1).
d to CLas. The promising candidate AMPs are then tested for toxicity to plants and animal cells. AMPs that pass these tests are then tested in HLB-affected citrus trees in the greenhouse and then in the field, to determine if they destroy CLas and enhance tree health. This process is outlined (Figure 1).Who is working on this project?
Goutam Gupta (The New Mexico Consortium), Supratim Basu (The New Mexico Consortium), Pankaj Travedi (Colorado State University), Joseph Krystel (USDA Agricultural Research Laboratory in Ft. Pierce, FL), Madhu Kunta (Texas A&M University at Kingsville), Randy Niedz (USDA Ft. Pierce), Joseph Patt (USDA Ft. Pierce), Surya Saha (Boyce Thompson Institute), Lukas Mueller (Boyce Thompson Institute), and Michael Braverman (Rutgers University).
What are our greatest challenges?
The team has completed one year of a three-year project. The latest generation of peptides is currently under evaluation. An emerging challenge is that application methods that demonstrate effective kill of CLas in greenhouse trees are not readily transferable to field-grown trees. An extensive portfolio of information on environmental, fruit quality, and other effects must also be assembled and assessed by EPA and FDA before these peptides will be available for use by growers.
Funding source: USDA- National Institute of Food and Agriculture (NIFA)- Citrus Disease Research and Extension Program
-
Developing novel biological delivery methods for therapeutic agents and other biomolecules to enhance production of citrus
Research by USDA Agricultural Research Service in Ft. Pierce FL, Ithaca NY, Albany CA, Wapato WA, and Dawson GA; Codex DNA in La Jolla CA; AgroSource Inc. in Tequesta FL; University of Florida in Ft. Pierce FL; Cornell University in Ithaca NY; and Indian River State College in Ft. Pierce FL
Article written by Robert Shatters, Ed Stover, Kimberly Wood
Article edited by Michelle Heck, Mark Trimmer, Peggy Lemaux, Lukasz Stelinski
Revised July 14, 2025.What is the research?
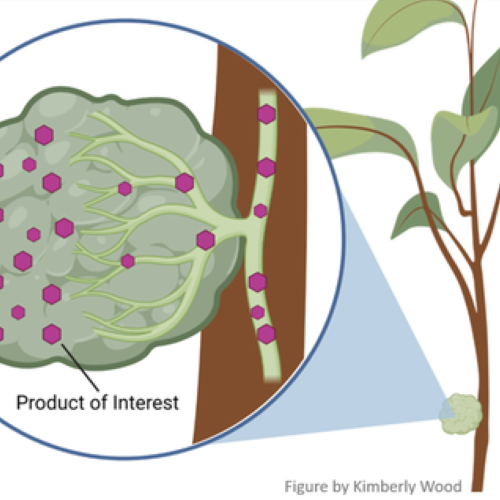
Scientific advancements have uncovered many ways to enhance crop-plant performance using new therapeutic approaches, but implementation in commercial agriculture has been slow. In this project, started in 2020 but building on several prior projects, delivery methods are being developed for cost-effective application in commercial agriculture. Several technologies are used, but a major focus is optimization of molecules in plant cells (biomolecules), known as SymbiontsTM, which are created through genetic manipulation. Symbionts, graft
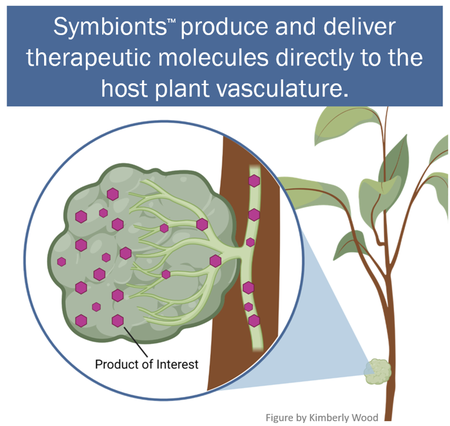 ed to crop plants (surgically attached to connect to plant tissues, see Figure) for biomolecule delivery throughout the plants, produce therapeutic molecules in a manner which optimizes their commercial benefits. Molecular biology methods are used to maximize biomolecule production and distribution to critical plant tissues. Agricultural engineers are developing methods for efficient grafting of Symbionts to citrus or other target plants in the nursery and field. In addition to direct production in plants, specially designed Symbionts, grown in liquid culture, produce biomolecules at high rates such that they can be cheaply extracted and used for direct plant application, like current agrochemical sprays. This NIFA-funded project has many complementary components such as: phloem targeted delivery devices; new HLB-suppressing therapies (including nanobodies); testing potential therapies from this and other projects in a bench to field pipeline; and HLB-targeted crop genetic engineering.
ed to crop plants (surgically attached to connect to plant tissues, see Figure) for biomolecule delivery throughout the plants, produce therapeutic molecules in a manner which optimizes their commercial benefits. Molecular biology methods are used to maximize biomolecule production and distribution to critical plant tissues. Agricultural engineers are developing methods for efficient grafting of Symbionts to citrus or other target plants in the nursery and field. In addition to direct production in plants, specially designed Symbionts, grown in liquid culture, produce biomolecules at high rates such that they can be cheaply extracted and used for direct plant application, like current agrochemical sprays. This NIFA-funded project has many complementary components such as: phloem targeted delivery devices; new HLB-suppressing therapies (including nanobodies); testing potential therapies from this and other projects in a bench to field pipeline; and HLB-targeted crop genetic engineering.What are some of the major successes to date?
Symbionts have been shown to alleviate citrus greening symptoms in small citrus trees in the greenhouse, and discussions with APHIS are underway to conduct field evaluations. Molecular biology methods have been used to refine the genetic manipulations in Symbionts to enhance biomolecule production. Symbionts grown in culture have produced high levels of biomolecule production reaching multi-gram quantities. These biomolecules are plant-based and can be delivered to citrus trees using trunk infusion.
The team has also engineered plants to express molecules that are toxic to Asian citrus psyllids with induced mortalities of up to 83% (vs. 5% in normal control plants) when fed on by adult psyllids. Engineered plants, designed to produce molecules that kill the bacterium that causes citrus greening disease, are currently under evaluation.
Who is working on this project?R, Shatters, R. Niedz, J. Krystel, M. Grando, W. Hunter, C. McKenzie, E. Stover from USDA Fort Pierce; C. Butts and J. McIntyre from USDA Dawson; R. Cooper from USDA Wapato; J. Thomson from USDA Albany; M. Heck, S. DeBlasio, S.T. Coradetti from USDA Ithaca; M. Trimmer, M. Pitino, L. Fleites from AgroSource; D. Gibson, K. Kannan from Codex DNA; L. Rossi, J. Qureshi from U Florida; M. Rivera from Cornell U; and T. D’Elia from Indian River State College.
What are the challenges and opportunities?
The team has completed 1.5 years of a five-year project. Tremendous progress has occurred on most fronts as the team works toward commercialization of the Symbionts, direct plant infusion of Symbiont-produced biomolecules, and transgenic strategies. Candidate therapies must demonstrate consistent performance under field conditions. In addition, an extensive portfolio of information on environmental, fruit quality, and other effects must also be assembled and assessed by regulatory agencies before therapeutic materials and Symbionts will be available for use by growers.
Funding source: USDA-National Institute of Food and Agriculture (NIFA) - Citrus Disease Research and Extension Program # Grant 2020-70029-33176
-
SP HLB-resistant rootstock candidates for the citrus industry: validating and understanding disease resistance
Research by: John Chater, Jude Grosser, Fred Gmitter, Liliana Cano, Yu Wang, Zhenyu Jia, and Manjul Dutt, all of the University of Florida
Article written by: John Chater
Article edited by: Ed Stover, Lukasz Stelinski, Peggy G. Lemaux
Revised July 14, 2025.What is the technique?
This project will investigate candidate rootstock selections that do not test positive for CLas, the bacterium that causes huanglongbing (HLB), and/or appear resistant to HLB by first testing positive and then testing negative, seemingly eliminating CLas (Figure 1). Experiments will be conducted on inoculated plants in the greenhouse and inarching (grafting new rootstocks into established trees) experiments that will be conducted in an infected grove. ‘Valencia’ scions infected with HLB will be grafted on the rootstocks. The rootstocks will include several selections that are showing some level of resistance plus two industry-standard rootstocks. This project will gather horticultural data and multi-omic data (described below) to gain knowledge about the complex interactions leading to HLB tolerance and resistance. Horticultural data collected will include traits important to citrus production, including plant growth, plant health, number and size of leaves and length and diameter of shoots. Data will also be collected on HLB symptoms and attempts will be made to quantify huanglongbing bacterial titer levels to determine infection levels. The multi-omics data that will be collected include genomic (relating to genome DNA), transcriptomic (relating to RNA levels), and metabolomic (relating to biochemistry and metabolism) data.
How is that procedure involved in the research being described and what was discovered?
Leaves and roots of the scion and rootstock, respectively, will be sampled at three time points each year, over multiple years, to determine differences in gene expression and metabolomic profiles among the rootstocks used in this study. Movement between rootstock and scion of metabolites or RNAs, which may be implicated in the HLB disease response, will also be evaluated. The project is still in its early phases in April 2023 and growers will be updated as new discoveries are made.
Who is working on this project?John Chater, Jude Grosser, Fred Gmitter, Liliana Cano, Yu Wang, Zhenyu Jia, and Manjul Dutt at the University of Florida are working on this project.
What are the challenges they are facing?Challenges faced include sifting through “big data” sets and using that information in multi-omics models. Making sure plants are propagated well and successfully inoculated with infected budwood are early challenges in this study. With multi-omics data, sometimes it is a challenge to identify certain components. For example, with metabolomics, there are often molecules that are unknown and unclassified.

Ungrafted Pummelo X C. latipes tree that has consistently been entirely HLB free. This accession displays tree health
as though not infected with the HLB pathogen. Because it is not testing positive, and it is displaying a relatively high level of tree health, this genotype is one of the potentially HLB-resistant rootstocks selected for study in this project.Funding source: United States Department of Agriculture, National Institute of Food and Agriculture
-
Advanced testing and commercialization of novel defensin peptides and therapies for HLB control
Research by Kranthi Mandadi, Texas A&M AgriLife
Article written by: Kranthi Mandadi, Sonia Irigoyen, Texas A&M AgriLife
Article edited by: Lukasz Stelinski, Peggy G. Lemaux,
Revised July 14, 2025.What is the technique?
Citrus greening or Huanglongbing (HLB), associated with Candidatus Liberibacter asiaticus (CLas) in the U.S., is the most devastating citrus disease today and has invaded most production areas in the U.S. Developing effective and commercially viable HLB control strategies is critical for the survival of the U.S. citrus industry. In this coordinated agricultural project, we are pursuing research and evaluation of promising HLB therapies and extending outcomes to stakeholders by leveraging public-private partnerships between State agencies, Universities, USDA-ARS, and the citrus industry
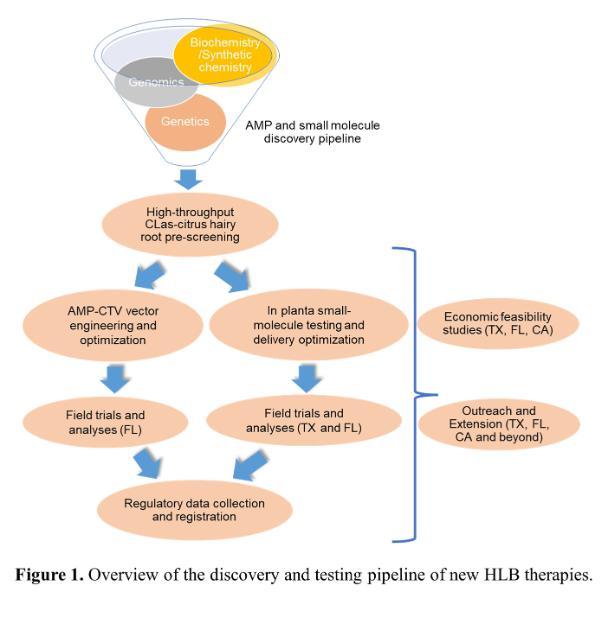 How are these procedures involved in the research described and what was discovered?
How are these procedures involved in the research described and what was discovered?To speed the discovery of HLB therapies, we developed an innovative CLas-citrus hairy root system that enables high throughput efficacy screening of antimicrobial peptides, small molecules, defense-related genes, and gene editing (CRISPR) targets for HLB management1. We are using this system to pre-screen two types of therapies: i) antimicrobial peptides (plant defensins) and ii) chemical compounds. Results so far have identified multiple peptides and chemical compounds with promising anti-CLas activity, which have been advanced to multi-location field trials using the Citrus tristeza virus vector and trunk injection-based delivery systems, respectively. Further economic feasibility studies will be conducted on the treatments that were shown to improve yield to determine the extent of benefit to citrus growers. A multi-state extension and outreach team uses various multi-media tools to disseminate project information to the stakeholders. Lastly, we are advancing promising therapies into a commercialization pipeline to seek regulatory approvals through industry partnerships.
Who is working on this project?Kranthi Mandadi, Sonia Irigoyen, Manikandan Ramasamy, Freddy Ibanez, Juan Enciso, Samuel Zapata, Olufemi Alabi (Texas A&M AgriLife, Texas A&M University); Veronica Ancona (Texas A&M University-Kingsville); Ute Albrecht, Choaa El-Mohtar, Ariel Singerman (University of Florida); Jinha Jung (Purdue), Hung Doan, Sonia Rios, Ben Faber, Greg Douhan (University of California); Ray Yokomi (USDA-ARS), and Mike Irey (Southern Gardens Citrus, U.S. Sugar).
What are the challenges they are facing?
The major hurdle, in our view, is the regulatory process and commercialization of effective therapies. The extensive timelines and costs (>$130 million) associated with deregulating and registering new HLB antimicrobials or delivery systems require strong partnerships and continued support from the private sector. This is particularly challenging today, given the declining citrus industry in Florida.
Funding source: USDA NIFA ECDRE (2021-70029-36056), Texas A&M AgriLife Research, Southern Gardens Citrus, U.S. Sugar
-
CAP: Combining Individual Protective Covers (IPCs) and brassinosteroids to prolong health and improve fruit yield and quality in newly planted trees under HLB
Research by Caroline Roper, Ph.D., Philippe Rolshausen, Ph.D., Danelle Seymour, Ph.D., Jason Stajich, Ph.D., Ashraf El-kereamy, Ph.D., Ute Albrecht, Ph.D., Sarah Strauss, Ph.D., Ramdas Kanissery, Ph.D., Dario Cantu, Ph.D. and Jonathan Kaplan, Ph.D.
Article written by: Caroline Roper, Ph.D.
Article edited by: Ed Stover, Lukasz Stelinski, Peggy G. Lemaux
Revised July 14, 2025.What is the research objective?
HLB is a major threat to US citrus production. It is highly destructive and lethal to commercial citrus cultivars making it the most serious current citrus disease. In the US, Candidatus Liberibacter asiaticus (CLas), is the primary bacterial species associated with HLB and the primary vector is the Asian Citrus Psyllid (ACP), Diaphorina citri. Despite years of research, there is no cure for HLB, and management practices focus on vector management/exclusion and maintaining tree health through nutrition regimes. Root collapse is an issue in HLB-impacted trees and a 2014 study suggested that CLas colonization leads to fibrous root decline prior to manifestation of above ground symptoms. The research objective of the proposed work is to investigate the root collapse associated with HLB-impacted trees and ways to mitigate it by promoting root health.
What is being done?This work builds on our previous work that demonstrated that, as HLB severity increases, the microbes associated with the root (microbiome) becomes enriched in soil-borne pathogens. We are conducting experiments to empirically determine if these path
 ogens exacerbate the HLB-associated root (and canopy) decline. We have integrated field studies that test HLB resistant/tolerant rootstocks and use of soil amendments that promote root health to determine if they suppress pathogenic shifts in the microbiome and prolong tree longevity/productivity under HLB pressure. These field studies include newly planted and established groves to investigate the efficacy of soil amendments and cover crops in both grove establishment and rehabilitation. To support our field trials and decipher potential mechanisms underlying the interaction of root health, the soil/plant microbiome and soil amendments we are also conducting microbial community analyses on above and below ground compartments of trees in our field trials. We are integrating this research with a robust extension and outreach program in combination with an economic cost-benefit analysis structured around adoption of treatments that enhance root health into commercial citriculture.
ogens exacerbate the HLB-associated root (and canopy) decline. We have integrated field studies that test HLB resistant/tolerant rootstocks and use of soil amendments that promote root health to determine if they suppress pathogenic shifts in the microbiome and prolong tree longevity/productivity under HLB pressure. These field studies include newly planted and established groves to investigate the efficacy of soil amendments and cover crops in both grove establishment and rehabilitation. To support our field trials and decipher potential mechanisms underlying the interaction of root health, the soil/plant microbiome and soil amendments we are also conducting microbial community analyses on above and below ground compartments of trees in our field trials. We are integrating this research with a robust extension and outreach program in combination with an economic cost-benefit analysis structured around adoption of treatments that enhance root health into commercial citriculture.Who is working on this project?
We have a broad team of academic faculty, scientists, postdoctoral scholars, graduate and undergraduate students working together to perform the work that underpins this project. Our techniques include in-field disease assessments and plant/soil sample collection in both California and Florida citrus groves. We are assessing root health below ground using a variety of metrics and specialized equipment, such as the rhizotron, which allows real time observation of root growth. At the microbial level, we are identifying and enumerating citrus root microbial communities by using metagenomics, which allows us to explain how microbial interactions are impacted by HLB. This work will inform us not only of the microbial populations enriched under the particular cultural practices we are testing but also predict their particular function as possible therapeutics for treating HLB.
Funding source: Unites States Department of Agriculture, National Institute of Food and Agriculture, Specialty Crop Research Initiative, Emergency Citrus Disease Research and Extension (#2020-70029-33202).
-
SP: Virus-induced gene selencing (VIGS) using insect-specific viruses to manipulate psyllids as a strategy to help control citrus greening/HLB
Research by: Diogo M. Galdeano, Curtis R. Carson, Emilyn E. Matsumura, Tanvi Rawat, William Ingram, Jun Jiang, Bryce W. Falk, and Yen-Wen Kuo
Article written by: Yen-Wen Kuo
Article edited by: Bryce W. Falk, Diogo M. Galdeano, Ed Stover, Peggy G. Lemaux
Revised July 15, 2025.
What is the technique?RNA interference (RNAi) is a biological mechanism where small RNAs bind to RNA and prevent the RNA translation process or where they degrade long RNAs with a specific targeted sequence, resulting in a change in gene expression and related metabolism. This mechanism was found to be essential not only for regulating gene expression in plants and animals, but also for host defense mechanisms. In this project, we are developing non-pathogenic virus-based RNAi induction to interfere with Asian citrus psyllid (Diaphorina citri) infection - with the goal of suppressing HLB. Applications, such as sprays of double-strand RNAs to induce RNAi in citrus, are expensive and inefficient. For sustained and effective solutions to block or suppress Candidatus Liberibacter asiaticus (CLas) in D. citri, we are developing a virus-based system using viral endosymbionts of D. citri. These endosymbionts are viruses that live in close physical association with plant cells and are in a situation in which both organisms benefit.
How is that procedure involved in the research?With improved DNA/RNA sequencing, scientists have discovered a diversity of viruses in insects, most of which are passed through generations, but induce no disease. Our group has identified multiple D. citri-specific viruses. The main goal for our current project is to complement HLB management efforts through manipulating the balance and/or interactions between D. citri endosymbionts, which are essential for insect metabolism and immune responses, to block acquisition and/or transmission of CLas (Figure 1). We are constructing infectious clones of four D. citri-specific viruses and evaluating effects of those viruses on D. citri populations, collected from California, Uruguay, and Taiwan. The variety of different types of viruses could be helpful for future applications in different D. citri populations and for different purposes. In addition to constructing viral infectious clones of the D. citri-specific viruses, we are studying the biology of these viruses to gain a fundamental understanding of this complex system to ensure that we use optimal approaches.
Who is working on the project?Researchers on this project include Diogo M. Galdeano, Curtis R. Carson, Tanvi Rawat, William Ingram, Bryce W. Falk, and Yen-Wen Kuo at the University of California, Davis, Emilyn E. Matsumura at the Wageningen University & Research in the Netherlands, and Jun Jiang, currently at Shandong Agricultural University, China.
What are the challenges?To develop a comprehensive and thorough insect-specific virus (ISV)-based management strategy against HLB, we have simultaneously studied the D. citri genome that provides an important foundation for future D. citri and citrus greening studies. The biology studies of D. citri and its viral endosymbionts will help us choose the best potential target sequences to achieve the desired effects on Clas transmission. The breadth of our D. citri genetic resources, D. citri viruses in culture, and the depth of data generated so far make us uniquely able to use viral endosymbionts as future tools for citrus greening biocontrol strategies.
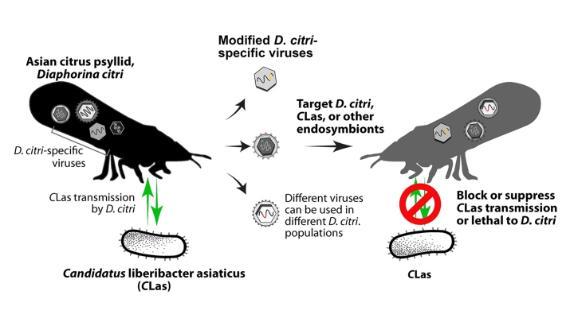
Figure 1. Main concept for Diaphorina citri-specific virus applications. The D. citri-specific viruses that were identified infecting D. citri (Asian citrus psyllid) in different geographical populations are being developed into viral vectors. Those viruses can vertically transmit to the progenies and spread into D. citri populations, to target D. citri, Candidatus Liberibacter asiaticus (CLas, causal agent of Huanglongbing), or other endosymbionts in D. citri. The targets will be designed to block or suppress CLas transmission or to be lethal to D. citri. Different viruses will be used for different D. citri populations based on our results from the biology assays
Funding source: United States Department of Agriculture-National Institute of Food and Agriculture (USDA-NIFA), Emergency Citrus Disease Research and Extension (ECDRE) Program
-
Phloem-restricted, independently mobile RNA gene silencing system for mitigating citrus greening (HLB) by targeting Liberibacter asiaticus and citrus gene expression
Research by: Dr. Anne Simon, Neena Mitter, Shunyuan Xiao, Silvec Biologics
Article written by: Anne Simon
Article edited by: Ed Stover, Peggy G. Lemaux
Revised July 16, 2025.What is the technique?
Bacterial, fungal and viral pathogens are causing the deaths of billions of trees and vines every year. It is widely accepted that RNA and peptide therapies offer solutions to controlling plant pathogens and the insects that deliver them, and such therapies are also hailed as the future of human medicine. Tiny RNAs known as siRNAs, which are naturally generated by all plants, get taken up by invading pathogens and pests where they target essential genes, thereby significantly reducing harmful pathogen effects. Numerous studies have demonstrated that plant siRNAs, whether natural or designed, are harmless when consumed by animals.
How is that procedure used in this research?Although siRNAs offer solutions to many agricultural problems, the problem of getting designer, protective siRNAs into a tree and spread throughout the canopy (without genetically engineering the tree) has been a major impediment for applying this technology to citrus. Although spraying and trunk injecting the siRNAs are possible, applications need to be repeated every few weeks making such treatments unsustainable, especially for long-lived trees. Viruses, however, are well known to be excellent vehicles (vectors) for generating copious amounts of designer siRNAs (and antibacterial peptides) throughout a tree, a technique known as virus-induced gene silencing (VIGS). While laboratory usage of VIGS in short-lived annuals has been routine for decades, VIGS vectors have not been stable, with most rapidly losing the ability to make designer siRNAs and peptides, significantly complicating applying this technology to trees.
We developed a natural citrus virus as a VIGS vector that is symptomless, has no discernable effect on fruit quality, and cannot be transmitted from tree-to-tree through pollen or seeds. Most importantly, we have discovered how to make our VIGS vector stable, allowing the protective effect to last a long time, hopefully for the life of a tree with a si
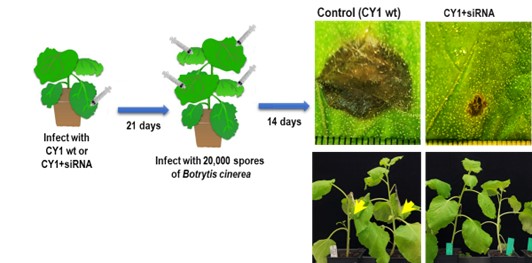 ngle application. For this project, we are modifying our VIGS vector to generate siRNAs that can target Candidatus Liberibacter asiaticus (CLas), and other funding is being used to target citrus pathogenic fungi, nematodes and other disease agents.
ngle application. For this project, we are modifying our VIGS vector to generate siRNAs that can target Candidatus Liberibacter asiaticus (CLas), and other funding is being used to target citrus pathogenic fungi, nematodes and other disease agents. Figure 1. VIGS vector CY1 with anti-fungal siRNA showing excellent protection
in laboratory plants against the fungus, Botrytis cinereaWho is working on this project?
The Simon lab at the University of Maryland leads the project, but collaborations with other labs have contributed technologies to enhance progress. While our VIGS vector can easily be introduced into seedlings (or bearing trees) through grafting, for more rapid dissemination in mature trees we are working with Dr. Neena Mitter, University of Queensland, on using her novel nanoparticle nucleic acid delivery method to introduce the vector as a stable spray throughout a tree’s canopy. The team also includes Dr. Shunyuan Xiao (IBBR/UMd), who is developing the anti-CLas siRNAs using the closely related Liberibacter crescens as a surrogate host, Michelle Heck (Cornell/USDA), who will test the VIGS vector in infected citrus trees, and Silvec Biologics, a company founded to commercialize VIGS vectors, and which has licensed the technology from UMd.
What are the challenges being facing?We are also engineering the VIGS vector to make an anti-bacterial peptide, as it is not known yet whether we can reach the levels of siRNAs needed to target bacteria. Several such peptides have already been developed that are extremely good at targeting CLas.
Funding source: USDA-NIFA ECDR 2022-70029-38492
-
CAP: Combining cultural and genetic approaches for grove success to unravel and enhance resistance/tolerance to Huanglongbing
Research by Caroline Roper, Ph.D., Philippe Rolshausen, Ph.D., Danelle Seymour, Ph.D., Jason Stajich, Ph.D., Ashraf El-kereamy, Ph.D., Ute Albrecht, Ph.D., Sarah Strauss, Ph.D., Ramdas Kanissery, Ph.D., Dario Cantu, Ph.D. and Jonathan Kaplan, Ph.D.
Article written by: Caroline Roper, Ph.D.
Article edited by: Ed Stover, Lukasz Stelinski, Peggy G. Lemaux
Revised July 15, 2025.What is the research objective?
HLB is a major threat to US citrus production. It is highly destructive and lethal to commercial citrus cultivars making it the most serious current citrus disease. In the US, Candidatus Liberibacter asiaticus (CLas), is the primary bacterial species associated with HLB and the primary vector is the Asian Citrus Psyllid (ACP), Diaphorina citri. Despite years of research, there is no cure for HLB, and management practices focus on vector management/exclusion and maintaining tree health through nutrition regimes. Root collapse is an issue in HLB-impacted trees and a 2014 study suggested that CLas colonization leads to fibrous root decline prior to manifestation of above ground symptoms. The research objective of the proposed work is to investigate the root collapse associated with HLB-impacted trees and ways to mitigate it by promoting root health.
What is being done?This work builds on our previous work that demonstrated that, as HLB severity increases, the microbes associated with the root (microbiome) becomes enriched in soil-borne pathogens (Figure 1). We are conducting experiments to empirically determine if these pathogens exacerbate the HLB-associated root (and canopy) decline. We have integrated field studies that test HLB resistant/tolerant rootstocks and use of soil amendments that promote root health to determine if they suppress pathogenic shifts in the microbiome and prolong tree longevity/productivity under HLB pressure. These field studies include newly planted and established groves to investigate the efficacy of soil amendments and cover crops in both grove establishment and rehabilitation. To support our field trials and decipher potential mechanisms underlying the interaction of root health, the soil/plant microbiome and soil amendments we are also conducting microbial community analyses on above and below ground compartments of trees in our field trials. We are integrating this research with a robust extension and outreach program in combination with an economic cost-benefit analysis structured around adoption of treatments that enhance root health into commercial citriculture.
Who is working on this project?We have a broad team of academic faculty, scientists, postdoctoral scholars, graduate and undergraduate students working together to perform the work that underpins this project. Our techniques include in-field disease assessments and plant/soil sample collection in both California and Florida citrus groves. We are assessing root health below ground using a variety of metrics and specialized equipment, such as the rhizotron, which allows real time observation of root growth. At the microbial level, we are identifying and enumerating citrus root microbial communities by using metagenomics, which allows us to explain how microbial interactions are impacted by HLB. This work will inform us not only of the microbial populations enriched under the particular cultural practices we are testing, but also predict their particular function as possible therapeutics for treating HLB.

Funding source: Unites States Department of Agriculture, National Institute of Food and Agriculture, Specialty Crop Research Initiative, Emergency Citrus Disease Research and Extension (#2020-70029-33202).
-
Endophytes as sources of antimicrobials to control Huanglongbing (HLB)
Research by: Dr. Kateel Shetty, Florida International University, Texas A&M University; University of Florida
Article written by: Kateel G. Shetty
Article edited by: Ed Stover, Peggy Lemaux, Lukasz Stelinski
What is the research?Endophytes are microorganisms which live inside healthy plant tissues, playing important roles in promoting plant growth and providing protection against pests and pathogens. Results from our research are focused on the microorganisms living in a specific environment of citrus plants, called the microbiome. In our case microbes are taken specifically from survivor trees. Our work showed that crude extracts of four bacteria were highly effective at killing live cells containing the HLB pathogen, CLas, in assays using ground extract of CLas-positive psyllids. The endophytic bacteria chosen were selected based on their activity against a culturable CLas substitute species, such as Liberibacter crescens. The antimicrobial activity was associated with the unconcentrated, cell-free culture extract, indicating that the unknown compound(s) is water- soluble and effective at low concentrations.
The overall goal of this project is to identify and test highly efficacious anti-CLas compounds derived from citrus endophytic bacteria that can be used to control HLB. In this USDA-NIFA-funded project we will focus on purification and characterization of the most active anti-CLas compounds from selected endophytic bacteria (Figure 1). These compounds will be pre-screened to identify the component/s that are the most active for anti-CLas activity, using the citrus CLas hairy root assay. They will be further evaluated for protective and curative effects of the purified antimicrobial compound/s against CLas under greenhouse conditions. Stakeholder outreach and engagement will be conducted through Cooperative Extension in Florida and Texas at different stages of the project.
Who is working on this project?Kateel G. Shetty, Krish Jayachandran, and Diego Salazar Amoretti from Florida International University (FIU); Kranthi K. Mandadi from Texas A&M AgriLife Research and Extension Center; Megan M. Dewdney from UF/IFAS Citrus Research and Education Center (CREC), and Amir Rezazadeh from UF/IFAS, Ft. Pierce.
What are the challenges and opportunities?The initial phase of the project, the extraction, fractionation, purification, and characterization of active anti-CLas compounds from individual endophytes is currently in progress. A new class of antimicrobials might require development of new chemical methods and protocols for separation and characterization of antimicrobial compounds. Combining biological compounds with different modes of actions against HLB should provide superior resistance approaches. Scaling up the production of antimicrobials and improving and optimizing plant delivery of antimicrobials may require additional time, effort, and approaches. Additional validation at the field level will be needed before the technology can be adopted for practical use. Marrone Bio Innovations, Inc., a commercial bio-pesticide company, based in California (https://marronebio.com/), has expressed interest in the project. Such public-private partnerships with commercial bio-pesticide companies can help bear the costs of the regulatory burden, commercialization, and product development needed to meet the needs of growers.

Figure 1. Process of Testing Anti-CLas Compounds
-
A collaborative approach between academics, growers and agrochemical industry to discover, develop and commercialize therapies for citrus Huanlgongbing (HLB)
Research by: Dr. Denise Manker, Biologics at Bayer Crop Science.
Article written by: Denise MankerArticle edited by: Ozgur Batuman, Ed Stover, Lukasz Stelinski, Peggy G. Lemaux
Article edited by: Peggy G. Lemaux, Ed Stover, Lukasz Stelinski
Revised July 16, 2025.What is the technique?
A screening cascade has been developed and validated with lab, greenhouse, and field tests to identify potential therapies with activity on HLB in citrus plants, as well as including development of HLB detection methods using metabolomics as an alternative to qPCR. This cascade has been used to test microbial strains and synthetic plant defense compounds that switch on the citrus trees’ immune system as well as other leads from start-up companies and other research groups. The goal of this research is to sift through many candidate therapies to find specific molecules and microbial strains that could be advanced towards registration for field application to improve health and yield of citrus trees with HLB.
Using this screening cascade, what has been discovered?The screening cascade is used to evaluate leads from collaborators and external parties. One new class of synthetic chemistry that turns on the citrus tree defense system has been demonstrated to significantly delay disease symptoms in the greenhouse and, based on qPCR, shows promise in two field trials (Figure 1). Confirmation is currently underway using analysis of citrus tree metabolites indicative of HLB infection. Several candidates have also shown potential for treating citrus canker.
 Figure 1. Greenhouse HLB assays at UF showing trunk injection sites in yellow for administering therapies to the phloem (left) and Field trials in Florida on new transplants with differences in vigor evident with various different treatments (right)
Figure 1. Greenhouse HLB assays at UF showing trunk injection sites in yellow for administering therapies to the phloem (left) and Field trials in Florida on new transplants with differences in vigor evident with various different treatments (right)
Who is working on this project?This Collaborative Agricultural Project has brought together research teams from the agrochemical industry and leading academic researchers. This includes the research labs of Bayer Crop Science in France, headed by Thomas Knobloch, working on new synthetic chemistries, Ginkgo Bioworks, formerly Bayer Biologics, working on microbial solutions led by Lorena Fernandez, Kranthi Mandadi’s team in Texas A&M AgriLife Research and Extension Center performing hairy root assay research, Ozgur Batuman’s lab at UF SWFREC developing and carrying out HLB citrus greenhouse testing, Cristina Davis’s lab developing metabolomic HLB detection and citrus health assays at UC Davis, and Mike Edenfield from Florida Agco carrying out multi-year field trials. The project director, Denise Manker, is with Bayer Biologics in the US.
What are the challenges we are facing?Identifying leads that can delay HLB infection or reduce infection in HLB-positive trees in the greenhouse and then translating the successful candidates to see if activity can be seen in field trials in growers’ groves has been the biggest challenge. This is because constant psyllid pressure in the field is difficult to replicate in greenhouse testing. As the citrus industry continues to change due to HLB and other challenges, maintaining ongoing trials given grove removals or finding field trial locations with new plantings, which will remain in place over several years, has also been a challenge.
Funding source: Initial funding (2017-2020) provided by CRDF with a supplement from CRB in 2020, followed by USDA NIFA CAP funding, Award 2020-70029-33196 starting in August 2020.
-
Developing tolerance to Huanglongbing disease in citrus by using gene editing
Research by: Zhonglin Mou, Ph.D., Jude Grosser, William Dawson, Vladimir Orbovic, Amit levy, Manjul Dutt, Choaa El Mohtar, Ozgur Batuman, and Michael Irey
Article written by: Dr. Zhonglin Mou
Article edited by: Ed Stover, Lukasz Stelinski, Peggy G. Lemaux
Revised, July 15, 2025.What is the research?
The overall goal of our research is to produce citrus varieties that are either resistant or tolerant to Huanglongbing (HLB). Our strategies are based on understanding the mechanisms that regulate the plant immune system. It is well-known that the plant immune system has both positive and negative regulators and the balance between the two can be shifted towards resistance or tolerance by adding positive or removing negative regulators. In this project we have engineered overexpression of more than 20 positive immune regulators in sweet orange and/or grapefruit. We found several immune regulators that conferred robust tolerance to HLB. Although these HLB-tolerant lines are valuable for the citrus industry, they are considered genetically modified organisms (GMOs). In this project, we attempt to generate non-GMO plants by creating HLB tolerance in commercial citrus varieties by silencing certain genes, using an editing technology termed CRISPR/Cas9 (see “New Genome Editing Technology” snapshot). These are not considered GMO under USDA regulatory laws. We first employ a delivery vehicle, based on the Citrus tristeza virus (CTV), that inserts the edits into citrus. By studying how tolerance happens in these GMO plants, we identify targets for our non-GMO editing approach to achieve the same tolerance outcome.
What are some of the major successes to date?
We developed CTV delivery vehicles for different editing strategies, that can shut down 21 different genes in citrus that control negative regulators in the citrus immune system. We evaluated how shutting down these negative regulators affect citrus plants, using a study system in the laboratory.
These plants are used to provide budwood for inoculation of commercial varieties with the editing CTV. All successful editing approaches have been graft-transmitted into sweet orange. The resulting trees were screened in the greenhouse for HLB tolerance. We have identified several gene targets that, when silenced, provide strong tolerance to HLB.
Meanwhile, a series of genome editing systems have been tested and vectors with high editing efficiency have been found. A number of different target genes have been edited and these edited plants are being screened for HLB tolerance currently
Figure 1. Both plants have been infected by the HLB-causing pathogen. The plant on the left is the control and the plant on the right is transgenic showing no or mild HLB symptoms.
Who is working on this projectZhonglin Mou, Jude Grosser, William Dawson, Vladimir Orbovic, Amit levy, Manjul Dutt, Choaa El Mohtar, Ozgur Batuman from the University of Florida; Michael Irey from United States Sugar Corporation Technical Operations.
What are the challenges and opportunities?We have successfully identified citrus genes encoding negative immune regulators that, when silenced, lead to strong HLB tolerance. We used various gene editing strategies to silence these genes to produce HLB-tolerant plants that are not considered GMO. While we have improved the editing efficiency, obtaining non-GMO edited plants is still a major challenge because current methods still leave DNA in the plants that make it a GMO. We are using new editing strategies to increase the chance of producing non-GMO edited plants.
Funding Source: NIFA ECDRE 2018-70016-27392
-
Development, evaluation and delivery of citrus HLB management approaches by targeting its nature as a pathogen-triggered immune disease
Research by: University of Florida, University of California Riverside, University of California Davis, Texas A&M University-Kingsville, Soilcea, Southern Gardens Citrus, LLC
Article written by: Nian Wang
Article reviewed by: Peggy G. Lemaux, Ed Stover, Lukasz Stelinski
Revised July 16, 2025.
What is the research?Recent work demonstrated that CLas stimulates a systemic and chronic immune response in the plant transport system (phloem) of HLB-sensitive citrus, causing cell death in the phloem and subsequent HLB disease symptoms (Figure 1). The hypothesis is that HLB symptoms are due to this systemic and chronic immune response and that the severity of the response can be controlled by reducing the reactive oxygen species (ROS), produced as a defense mechanism in the response to the HLB-pathogen
Genome editing (https://ucanr.edu/sites/scienceforcitrushealth/Research_Snapshots/General_Topics/LemauxCRISPR/) will be used to generate non-GMO citrus with reduced immune responses, resulting in HLB-resistant/tolerant citrus varieties. We developed tools for generating gene-edited citrus plants using special lab-cultured plant cells. This approach has been successfully used to generate non-GMO canker-resistant citrus and will be used here to generate non-GMO HLB-resistant/tolerant citrus varieties for use in new plantings. For established HLB-infected groves, genes will be expressed in trees for making antioxidants, that are introduced using the Citrus tristeza virus (CTV). This virus is already present in many commercial citrus groves but the strain we use produces minimal symptoms and in our work was modified to express genes to protect against HLB. It has been shown that such modified CTVs can express a foreign gene for more than a decade in citrus and such modified CTVs have already been given regulatory approval to deliver antimicrobial peptides in commercial citrus groves in Florida.
What are the objectives of this research?We will control HLB using three approaches. (1) Develop integrated horticultural approaches to mitigate CLas-triggered ROS production, which will involve optimized applications of micronutrients, gibberellic acid (GA) and antioxidants to lessen HLB symptoms. (2) Protect citrus plants from CLas-triggered ROS by expressing antioxidants and silencing genes involved in CLas-triggered ROS production. (3) Generate non-GMO HLB-resistant/tolerant citrus using editing of genes required for HLB disease.
Who is working on the project?Nian Wang of the University of Florida (UF); Danelle Seymour of the University of California, Riverside (UCR); Abhaya Dandekar of UC Davis; Madhurababu Kunta of Texas A&M University-Kingsville; Dusica Coltrane of Soilcea, LLC; Jude Grosser of UF; Ashraf El-Kereamy of UCR; Choaa El-Mohtar of UF; Zhengfei Guan of UF; Davie Kadyampakeni of UF; Martha Orozco-Cardenas of UCR; Chris Oswalt of UF; Tripti Vashisth of UF; Yu Wang of UF; Mongi Zekri of UF; and Mike Irey of Southern Gardens Citrus, LLC.
What are the challenges?Genome editing technology, using CRISPR, has been successfully used to generate non-GMO canker resistant citrus varieties but remains a laborious process with low throughput, and needs to be conducted separately on each cultivar of interest. The CTV technology described will provide a potential management approach to control HLB-diseased trees in the field. Each modified CTV will likely be useful across most citrus cultivars; however, CTV cross-protection, which hinders new infections in CTV-infected trees, may prevent use on many field trees that are already infected with CTV. State regulatory agencies may also require use of CTV strains that are already widespread in each state.
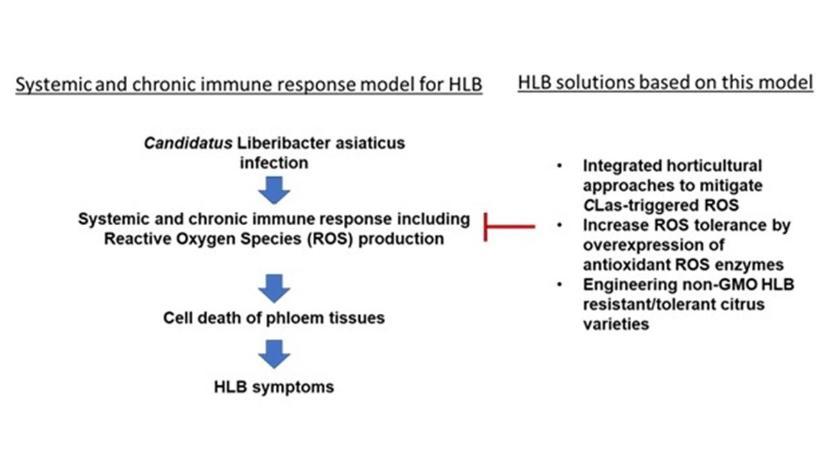 Figure 1. Elements of the systemic and chronic immune response model for HLB and associated solutions based on this model.
Figure 1. Elements of the systemic and chronic immune response model for HLB and associated solutions based on this model.Funding source: USDA NIFA ECDRE program, project #2022-70029-38471
-
Use performance of 300 hybrids in established trials to map Huanglongbing tolerance/resistance genes and release superior new rootstocks
Research by: USDA-ARS in Ft. Pierce, FL; University of California, Riverside in Riverside, CA, University of Florida in Wimauma, FL and Immokalee, FL
Article written by: Danelle Seymour
Article edited by: Kim Bowman, Peggy G. Lemaux
Revised July 15,2025.What is the research?
The USDA rootstock breeding program has previously identified several rootstocks with tolerance to HLB that are significantly more productive than other industry standard rootstocks in HLB-endemic environments. However, the current best HLB-tolerant rootstock still suffers a yield loss when diseased and further gains in tolerance or resistance to HLB are needed to sustain the industry. Field citrus trees in Florida are now uniformly infected with Candidatus ?Liberibacter asiaticus (CLas), providing an opportunity to screen thousands of trees for the ability to yield high quality fruit and juice in the presence of HLB. Beginning in 2014, a series of replicated field trials across three HLB-endemic growing regions in FL were initiated by the USDA rootstock breeding program (Figure 1). These trials now include over 300 new rootstock hybrids that have been evaluated in replicated field trials for up to eight years. Traits associated with field performance, fruit and juice quality, and disease progression in the common sweet orange scion have been measured yearly across more than 6,000 trees. Ultimately, this material will deliver new HLB-tolerant rootstocks to growers. In addition, the scale of these trials is being leveraged to dissect genetic control of tolerance or resistance to HLB in order to boost the efficiency of citrus rootstock breeding in the future.
What are major successes to date?
Several rootstock selections have been made that were identified with superior performance relative to industry standards. These selections have been submitted to clean budwood programs to facilitate their testing across different environments to determine if the productivity and overall performance observed in these trials hold up in multiple locations. Additionally, we have been able to demonstrate that there is genetic control of rootstock-mediated HLB tolerance, with substantial heritability observed in traits related to yield, tree size, and disease progression. This is good news for selective breeding for tolerance and/or resistance to HLB, indicating that breeders can continue to improve levels of rootstock-mediated tolerance to HLB.
Who is working on this project?
Kim Bowman from the USDA-ARS in Ft. Pierce, Florida, Danelle Seymour and Philippe Rolshausen from the University of California, Riverside, and Ute Albrecht and Zhanao Deng from the University of Florida.
What are the challenges and opportunities?
There is strong HLB disease pressure in Florida, which enables large-scale field testing for HLB-tolerance or resistance that is impossible in other regions. As a result, several promising new rootstock hybrids with superior performance under HLB-endemic conditions were identified in the trials established in 2014. We will continue to evaluate trials planted at later dates and expect additional rootstock hybrids with enhanced HLB tolerance to be identified. Because these hybrids have been evaluated in Florida only, although often in one or two locations, it is a challenge to predict their performance in other regions, each with unique biotic and abiotic stress conditions. In the future, we hope to initiate focused testing of rootstock selections for region-specific stresses so that recommendations can be tailored to the needs of citrus growers in each region.

Funding source: USDA-National Institute of Food and Agriculture (NIFA) - Citrus Disease Research and Extension Program Award # 2021-70029-36052
-
A bench to field pipeline for controlling citrus greening disease: A USDA NIFA Project
Research by USDA Agricultural Research Service
Article written by: Michelle Heck, Robert Shatters, Jr. and Randall Niedz
Edited by: Mark Trimmer, Cody Estes, Robert Adair, Peggy G. Lemaux, Lukasz Stelinski, and Ed Stover
Revised July 15, 2025.From science to solutions – project overview
Citrus greening is a complex, often fatal disease affecting citrus production. This disease is caused by a bacterium (CLas), which is carried by the Asian citrus psyllid. Bacteria-killing therapies, boosting plant immunity and/or preventing CLas transmission by psyllids all have potential to prevent CLas infection. This project aims to develop viable commercial solutions to citrus greening disease with a strong focus on developing delivery methods. Our pipeline contains molecules that we discover and also those that we solicit from the broader community. Our scientists can evaluate molecules by injecting them directly into the tree to see if the molecules have an effect and move systemically. We also test them by using ‘Symbionts’, a new technology for delivery we developed, which is described below.
What are Symbionts and how do they work?
Symbionts are a patented, revolutionary new plant biotechnology tool, similar to an insulin pump in that they deliver molecules directly into the tree. Symbionts are biological, connected to and developing with the tree during the growing season. Symbionts, which can be used in both established groves and new plantings, produce and deliver therapeutic molecules directly into citrus trees to improve tree health. Trees are not genetically altered in any way by Symbionts. Preliminary experiments with Symbionts show promise in the greenhouse and field trials will soon be underway. An innocuous strain of Agrobacterium, a natural soil dwelling bacterium, is the organism that we use to create Symbionts.
Why bypass lab assays and inject molecules directly into the tree?
The citrus industry in Florida is in a steep decline, and growers need an immediate solution. Scientists have made tremendous
progress
developing assays to identify compounds that kill CLas in the lab, but there is little evidence whether these molecules will enhance tree health in the field. Our team has made a comprehensive list of molecules, obtained large quantities of these molecules and are injecting trees to screen for their ability to greatly reduce citrus greening disease in commercial groves. This aspect of the project synergizes with the Symbiont work and we hope will provide growers with a more near-term solution with rapid EPA review and approval.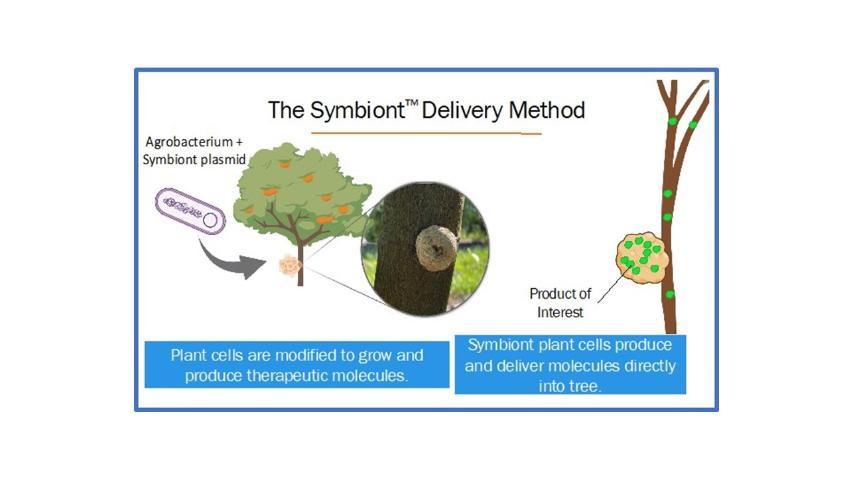
Who is currently working on this project?
Michelle Heck (Co-Project Director), Stacy DeBlasio, Luke Thompson, David Igwe and Samuel Coradetti (USDA ARS, Ithaca, NY); Robert Shatters (Project Director), Randall Niedz, Wayne Hunter, Joe Krystel Nick Larson, Douglas Stuehler and Cindy McKenzie (USDA ARS and ORISE, Fort Pierce, FL); Robert Adair (Florida Research Center for Agricultural Sustainability, Vero Beach, FL); John Fitzgerald, Laura Fleites, Doug Harper, Marco Pitino, Samantha Sullivan, Mark Trimmer and Patrick Zagorski (AgroSource, Inc.); Lorenzo Rossi, Ellen Cochraine and Guilherme Locatelli (UF-IFAS, Fort Pierce, FL); Jim Thomson (USDA ARS, Albany, CA), Ali Nookmazar, Dan Gibson and Krishna Kannan (Telesis Bio); Monique Rivera (Cornell); Cody Estes (Estes Citrus Inc, Vero Beach, FL); Rodney Cooper (USDA ARS, Wapato, WA)
What are our greatest challenges?
Developing the bench to field pipeline for evaluation of therapies took substantial effort to select delivery methods. Standard injection works well on field trees but is too damaging to use on small greenhouse trees, which must be used for efficient testing of numerous therapeutic compounds. For initial tests, we developed a 3-D printed device which delivers molecules to potted citrus trees. Our team overcame major challenges in the greenhouse evaluation of Symbiont technology. We have shown that Symbionts: do not impair plant health or productivity; can be produced rapidly on citrus (in one month); and can mitigate citrus greening in potted trees when used to deliver high impact therapeutic molecules. The greatest and most important challenge is conducting whole-tree evaluations in the field where there are many challenging variables. We are very fortunate to have grower cooperation and the assistance of the USDA APHIS and the EPA for field trials.
-
A Method for generating optimal attractive scents for Asian Citrus Psyllid (ACP) biocontrol
Research by: University of Connecticut and University of Florida
Article written by: Dr. Alexander Aksenov
Article edited by: Ed Stover, Lukasz Stelinski, Peggy G. Lemaux
Revised July 16, 2025.What is the research?
Frequent insecticide sprays have been used to suppress the HLB-pathogen’s insect vector, the Asian citrus psyllid (ACP). A far better strategy would be to use chemical lures to capture and kill the insects. Unfortunately, pheromones, secreted chemicals that act like hormones to affect the behavior of other insects, do not appear to attract ACP. Complex mixtures of volatile organic compounds (VOCs), mimicking citrus plant aromas, show some promise as attractants, but insect behavior changes with minor shifts in specific concentrations of these compounds. Using simple mixes of VOCs are not sufficiently attractive to ACP and controlled dispersal of more than a few compounds is technically challenging. To address this situation, we will optimize complex ACP-attractant VOCs, dispersed via newly developed materials, made out of a polymer support material interspersed with graphene (a conductive material made of a single layer of carbon atoms), that can capture and fix the VOCs. This material can deliver flexible, controlled mixtures of VOCs to optimize their attractiveness and can be used to disperse ACP attractants in citrus orchards. The goal of this research is to improve the attract-and-kill (AK) approach for ACP by optimizing the attractant, using the approach described below.
What are some of the major successes to date?Stelinski and colleagues developed an AK trap, using ACP-sensory responses, that are based on their look, feel and taste, and optimized trap components in greenhouse and simulated field conditions. Multiple sensory cues are used to attract psyllids to a cylindrical trap that has visible and UV colors, chemical attractants and compounds that attract pests and also contain a toxicant. The trap will be enhanced with a stimulant that mimicks complex citrus scents and that is dispersed using a novel volatile-release device with a single layer of carbon atoms (graphene), developed by the Adamson group (Figure 1). The graphene surface acts as an adsorber that controls compound release. Short current pulses are passed through the graphene, to heat the surface precisely to achieve controlled release of the compounds. By utilizing multiple graphene capsules, each with a controller, release of each compound can be precisely controlled.
Who is working on this project?Alexander Aksenov, Douglas Adamson, Ali Bazzi from the University of Connecticut; Lukasz Stelinski from the University of Florida.
What are the challenges and opportunities?The team has completed 0.5 years of a two-year project. We optimized the graphene composite manufacturing procedure to ensure consistent, reproducible properties. We extensively characterized release of individual VOCs from graphene. An initial, beta version of the device is being assembled to begin testing attraction on live insects. An anticipated challenge will be delivering sufficient numbers of devices per area of crop that can manage ACP populations under natural conditions. Manufacturing these materials could be scaled to produce an economically viable product because the cost of materials is relatively low.
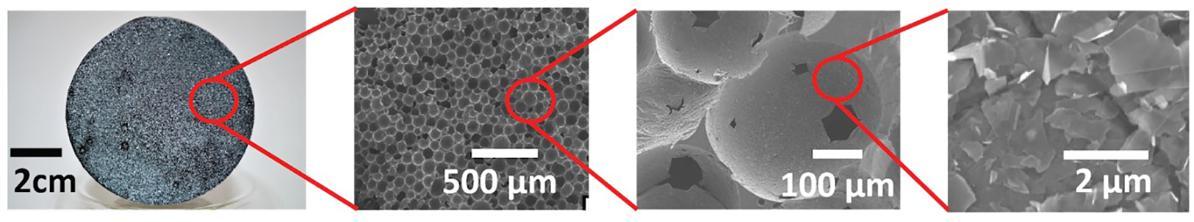
Figure 1. Novel, low-cost graphene composite at progressively greater magnification. Starting from the rigid open cell foam on the left, greater magnification reveals the foam's individual cells after removing the water. Further magnification shows holes or "windows", that create a porous network that connects to cells underneath. Final magnification (far right) shows individual graphene sheets on the interior surface of a cell.
Funding source: USDA-National Institute of Food and Agriculture (NIFA) - Citrus Disease Research and Extension Program # Grant USDA-NIFA-SCRI-009111