Psyllid Management
The snaphots on this page describe methods that could be used to alter or affect the psyllid so that the spread of HLB bacterium is suppressed.
-
Two-Pronged Approach to Suppress the Asian Citrus Psyllid Vector of HLB
Research by Dr. Bryony C. Bonning, University of Florida
Article written by Bryony C. Bonning; edited by Lukasz L. Stelinski and Peggy G. Lemaux
Revised July 7, 2025.What is the technique?
The Asian citrus psyllid (ACP) inoculates citrus trees with the CLas bacterium that causes huanglongbing (HLB). Effective tools to reduce psyllid populations are important for management of this complex disease. Certain pesticidal proteins, produced by the soil-borne bacterium Bacillus thuringiensis (Bt), are toxic to ACP. A next step in developing effective strategies against ACP was to test the effects of these pesticidal proteins in combination with gene silencing RNAs, small molecules that prevent expression of targeted ACP genes, essential for psyllid survival. Importantly, both the pesticidal proteins (Jurat-Fuentes and Crickmore 2017) and the gene silencing RNAs are not harmful to nontarget organisms.
How would this work?
Although the reason for this effect is currently unclear, Bt pesticidal proteins and gene silencing RNAs have been shown to be significantly more
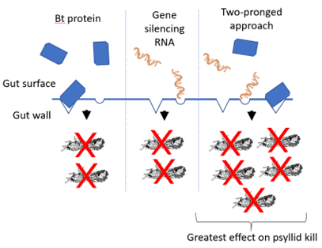 effective when used in combination than individually
effective when used in combination than individually
(Figure 1). Bt proteins and gene silencing RNAs are individually effective tools for killing Asian citrus psyllid. An approach combining the two techniques for increased effectiveness is shown. First, when ingested by ACP, the Bt pesticidal proteins bind to the wall of the insect gut and create a hole. The insect stops feeding as a result of the damage and ultimately dies. Second, the gene silencing RNAs shut down the targeted “survival” genes in the ACP gut so that production of proteins from those genes is blocked. One possible explanation for the enhanced combined effect is that the damage caused by the pesticidal Bt protein makes it easier for the gene silencing RNAs to enter into cells lining the psyllid gut.
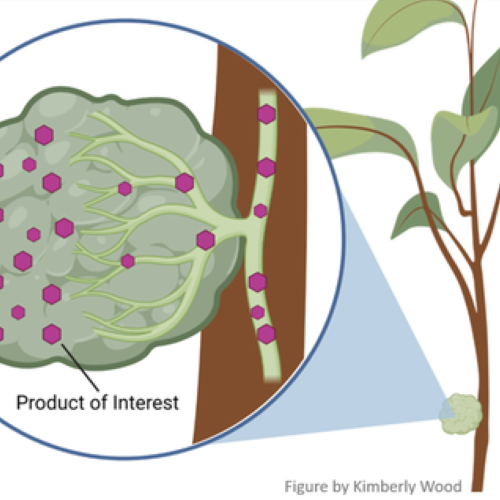
Disease management applicationsWhen the best combination of Bt pesticidal proteins and gene silencing RNAs has been identified, an appropriate delivery system to ACP will be needed. To be effective, both control agents need to be in the plant sap so that they are ingested as the psyllid feeds. One option is to use a naturally occurring plant virus (Citrus tristeza virus), that is known to reside in plant sap, for delivery of these agents. A second option is to engineer the citrus plant to make these compounds or to put them in a trap plant such as Indian curry that is more attractive to psyllids than fr
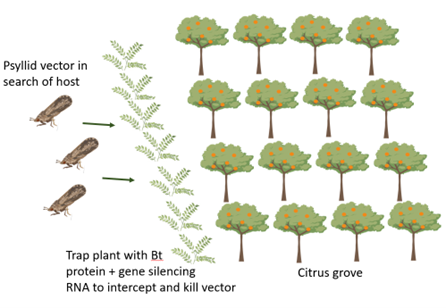 uit-producing citrus. In that situation, psyllids attracted to trap plants are killed by the combined Bt pesticidal protein and gene silencing RNAs before they are able to reach fruit-producing citrus trees. Also, the engineered genes would not be present in those citrus trees.
uit-producing citrus. In that situation, psyllids attracted to trap plants are killed by the combined Bt pesticidal protein and gene silencing RNAs before they are able to reach fruit-producing citrus trees. Also, the engineered genes would not be present in those citrus trees.One potential method to deploy this technology to protect commercial citrus groves is trap crops expressing Bt and gene silencing RNA, which intercept and kill the mobile ACP before they invade groves (Figure 2).
Who is working on the project?
Professor Bryony Bonning, leading the team based at University of Florida (UF), is conducting research on the Bt pesticidal proteins active against Asian citrus psyllid. This is in collaboration with Nabil Killiny, Associate Professor, Choaa El-Mohtar, Research Assistant Scientist, and Lukasz Stelinski, Professor, all at UF. The latter are examining the impacts of combinations of pesticidal proteins and gene silencing RNAs on ACP. Manjul Dutt, Research Assistant Scientist at UF, is assessing the best strategies for production of Bt proteins and silencing RNAs in citrus sap.
What are the challenges and opportunities?
A key challenge for this strategy is appropriate and effective delivery to ACP of both the pesticidal protein and gene silencing RNAs. Delivery of both components has been achieved for another major insect pest, the corn rootworm, and should therefore be feasible for targeting Asian citrus psyllid, but optimization for adequate production of both components in plant sap will be required. An additional approach would be to increase the toxicity of the pesticidal proteins thereby reducing the amount of protein that needs to be produced by the plant. Bonning’s lab is using an approach that creates an artificial anchor to increase the amount of protein that binds to the gut wall to increase its negative impact on ACP.
Jurat-Fuentes, JL & N.Crickmore (2017) Specificity determinants for Cry insecticidal proteins: Insights from their mode of action J. Invertebr. Pathol. 142:5-10. 10.1016/j.jip.2016.07.018
Funding source: This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2020-70029-33177. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
-
Artificial border fencing and live windbreaks for ACP management
Research by Dr. Mamoudou Sétamou, Texas A&M University Kingsville, and Dr. Xavier Martini, University of Florida
Article written by Mamoudou Sétamou, Xavier Martini, Monique Rivera, Elizabeth Grafton-Cardwell, and Peggy G. Lemaux.
Revised July 10, 2025What is the technique?
To date, heavy use of insecticides for management of Asian citrus psyllid (ACP), Diaphorina citri, has not prevented citrus greening or Huanglongbing (HLB) spread. Exclusion of ACP is the best approach to prevent ACP infestation of citrus groves as it effectively prevents the pest from coming into contact with citrus trees and establishing its populations. ACP prefers to live on the edges of groves and so the border trees are the first to be colonized. In addition, ACP adults move between habitats on a daily basis. These innate behaviors can be exploited to protect citrus by placing barriers around grove borders to prevent invasion and establishment of its populations.
ACP-resistant barriers can be either living windbreaks or artificial screen barriers, established around grove borders. Screen barriers should be built with a fence at least 12 ft high, 30-40 ft away from the border trees with psyllid resistant screens (Figure 1). It is well established that most psyllids are trapped when fences are between 2 and 10 ft. high; hence, a 12 ft. high fence will effectively exclude most psyllids from entering groves. In a field study conducted in Texas, border fences established along the southern and eastern borders of a grapefruit grove resulted in 55-98% reduction of ACP numbers relative to a control grove without border fences. The windbreak must be made of mesh and open to wind flow to avoid turbulence, which reduces efficacy.

Living windbreaks also reduce wind and ACP movement into the grove. They also reduce soil erosion, increase water retention and filter pollutants. They can also be used as refuges by beneficial insect species, such as pollinators and natural enemies. The ideal windbreak tree is an evergreen, long-lived and fast-growing tree that can grow at least 12 feet in height. It should be inexpensive, easy to establish and require only minimal maintenance. Finally, it is important not to select a tree that could function as an alternative host for citrus pests or diseases (citrus and other plants in the Rutaceae family).

Ideally, windbreaks should surround each side of the citrus grove. If, for economical or logistical reasons this is not feasible, it is wise to position the borders perpendicular to the main wind current during the year. Wind direction in a given area can be found on www.windhistory.com.
How do border fences improve ACP and HLB management?
Non-chemical control approaches play an increasingly important role in ACP management programs. Border mesh fences reduce the number of HLB-infected ACP flying into groves and thus reduce the risk of HLB spread. Windbreaks can be implemented either as a standalone approach or coupled with other management strategies such as pesticide sprays.
Research on living windbreaks significantly decrease the density of ACP on the border of citrus groves (Martini et al. 2015). They act mostly as physical barriers; however, they may also provide refuge for natural enemies of ACP if appropriate flora attractive to these species is present (Patt and Rohrig 2017, Patt 2018).
Who is working on this project?
Drs. Mamoudou Sétamou (Texas A&M University-Kingsville), Xavier Martini, Lauren Diepenbrock, and Lukasz Stelinski (University of Florida), Beth Grafton-Cardwell and Monique Rivera (University of California Riverside) are collaboratively exploring both ways to develop border management strategies.
What are the challenges and opportunities?
The deployment of border fencing and living windbreaks generally requires dedication of space (30-40 ft.) to establish the border wall and to allow for access of farm equipment between the border and crop edge. This may reduce the cropping area and competition between the crop and living windbreak trees should be considered. In some areas, grove borders are immediately adjacent to other crops, e.g., avocadoes, thus requiring the removal of producing trees before these border management approaches can be deployed.
Other challenges are the initial cost of and the time required for establishment of border mesh fencing. Although living windbreaks can be the cheapest and longest-lasting option, these trees take time to grow of sufficient height to prevent grove infestation by ACP, depending on the species of live windbreak chosen and the environment. In this case it is estimated to be at least five years.
Citations:
Lewis-Rosenblum, H., X. Martini, and S. Tiwari. 2015. Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. J. Econ. Entomol. 208: 3–10. (link)Martini, X., K. S. Pelz-Stelinski, and L. L. Stelinski. 2015. Absence of windbreaks and replanting citrus in solid sets increase density of Asian citrus psyllid populations. Agric. Ecosyst. Environ. 212: 168–174.(link)
Patt, J. M. 2018. Occurrence of coccinellids that prey on Diaphorina citri (Hemiptera: Liviidae) on Euphorbia heterophylla (Euphorbiaceae) and Chamaecrista fasciculata (Fabaceae) in a south Florida residential area. Florida Entomol. 101: 131–134. (link)
Patt, J. M., and E. Rohrig. 2017. Laboratory evaluations of the foraging success of Tamarixia radiata (Hymenoptera: Eulophidae) on flowers and extrafloral nectaries: potential use of nectar plants for conservation biological control of Asian citrus psyllid (Hemiptera: Liviidae). Florida Entomol. 100: 149–157. (link)
Sétamou, M., O. J. Alabi, N. Tofangsazi, and E. Grafton-Cardwell. 2018. COPF: Citrus orchard perimeter fencing as a strategy for reducing Asian citrus psyllid (Hemiptera:Liviidae) infestation. J. Appl. Entomol. 142: 959–966. (link)
Funding source: We gratefully acknowledge the financial support provided by the Texas Citrus Producers Board that assisted in covering partial labor costs of this project.
-
Using particle films to manage AC
Research by Dr. Christopher Vincent, University of Florida
Article written by Christopher Vincent, Monique Rivera, Elizabeth Grafton-Cardwell, & Peggy G. Lemaux.
Revised July 10, 2025.What is the technique?
Particle films are fine particles that are applied to plants to form a thin residue, or “film,” over the leaf surface. These films reflect light, giving the plant a bright or white appearance. Usually they are sprayed in a water suspension through a standard sprayer. The most common type of particle film is made from natural kaolin clay, a soft, white aluminum silicate, that is mined and filtered for a small particl
 e size. Another type of film consists of diatomaceous earth. The particles are applied to crops to manage environmental challenges, like high heat or sunburn, or manage pests, such as aphids. Particle films enhance plant growth under most conditions by helping the plants achieve a more optimal light distribution. The particles reflect light away from the outer leaves which keeps them from experiencing high light stress which, in turn, helps to reduce water loss and keep leaf temperatures within an optimal range. Some of the light reflected by the particle film appears to be redistributed deeper into the canopy which would assist more of the tree’s leaves to have access to light. Particle films reduce infestations by some pests because the particle films hide the natural plant colors that help some insects find their plant host. Recently we began field-testing mixes that use a particle film (Surround WP), made from kaolin that has an added red dye to improve the effect on pest management. Red enhances the pest management effect by reducing reflectance of yellow, blue, and ultraviolet light, which attract Asian citrus psyllid (ACP), the vector of HLB (Figure 1).
e size. Another type of film consists of diatomaceous earth. The particles are applied to crops to manage environmental challenges, like high heat or sunburn, or manage pests, such as aphids. Particle films enhance plant growth under most conditions by helping the plants achieve a more optimal light distribution. The particles reflect light away from the outer leaves which keeps them from experiencing high light stress which, in turn, helps to reduce water loss and keep leaf temperatures within an optimal range. Some of the light reflected by the particle film appears to be redistributed deeper into the canopy which would assist more of the tree’s leaves to have access to light. Particle films reduce infestations by some pests because the particle films hide the natural plant colors that help some insects find their plant host. Recently we began field-testing mixes that use a particle film (Surround WP), made from kaolin that has an added red dye to improve the effect on pest management. Red enhances the pest management effect by reducing reflectance of yellow, blue, and ultraviolet light, which attract Asian citrus psyllid (ACP), the vector of HLB (Figure 1).How are particle films used to manage HLB?
Particle films can dramatically r
 educe arrival of ACP. In our experimental plots in Florida, under high disease and ACP pressure, both natural white and red-dyed kaolin reduced ACP populations by more than 80% as compared to monthly insecticide treatments. Red kaolin produces a small reduction (4-5%) in ACP populations, relative to the white. Both treatments delayed HLB infection; the first detected infection in the red treatment was nearly 1 year after the first detected infections in the untreated trees. Both treatments have had positive effects on young tree growth, with kaolin-treated tree trunks having a nearly doubled girth versus untreated trees and much larger and denser canopies. Several growers are using kaolin regularly and report positive results in terms of tree health and growth (Figure 2).
educe arrival of ACP. In our experimental plots in Florida, under high disease and ACP pressure, both natural white and red-dyed kaolin reduced ACP populations by more than 80% as compared to monthly insecticide treatments. Red kaolin produces a small reduction (4-5%) in ACP populations, relative to the white. Both treatments delayed HLB infection; the first detected infection in the red treatment was nearly 1 year after the first detected infections in the untreated trees. Both treatments have had positive effects on young tree growth, with kaolin-treated tree trunks having a nearly doubled girth versus untreated trees and much larger and denser canopies. Several growers are using kaolin regularly and report positive results in terms of tree health and growth (Figure 2).Who is working on this project?
This project is being conducted by several people in the Tree Ecophysiology Laboratory at the University of Florida Citrus Research and Education Center, with help from Ed Etxeberria and Pedro Gonzalez at the same center. Additionally, a number of growers are experimenting with ways to apply kaolin more effectively.
What are the challenges and opportunities?
The major challenge in Florida is the ability to withstand rainfall. Frequent rains tend to wash off particle films, which are only effective as long as they cover the entire leaf. To maintain constant coverage in Florida we have had to apply every two weeks on average, and growers have noted similar frequencies, which drives the cost up. In California, growers use this product for sunburn protection and have commented that in desert conditions, they have difficulty adhering the product to new foliage. For these reasons, research is needed to develop formulations that adhere to the leaves better. Additionally, these films have disrupted natural enemies and led to California red scale outbreaks. To minimize this problem, we intend to test the efficacy of border treatments where the psyllids are most commonly found. Major opportunities are that particle films are appropriate for both organic or conventional use, and that they can effectively decrease ACP and delay HLB infection, while simultaneously enhancing growth.
Funding source: This project is funded by the Citrus Research and Development Foundation.
-
Attractants and traps for ACP management
Research by Dr. Lukasz Stelinski, University of Florida
Article written by Lukasz Stelinski, Elizabeth Grafton-Cardwell and Peggy G. Lemaux.
Revised July 10, 2025.What is the technique?
Attractants for insect pests of agricultural crops play an increasingly important role in management programs. Chemical, visual, and auditory cues that attract insects are identified during laboratory investigations and then verified for their attractiveness in the field. These cues are then exploited to serve as lures deployed with traps to attract pests coming from potentially long distances. This allows detection and monitoring of target pests and/or attracting and killing them t
 o reduce their numbers in the orchard.
o reduce their numbers in the orchard.
Traps may be coated with a sticky substance to ensnare individuals (Fig. 1A) or the insect may be trapped within an enclosure containing a killing agent (Fig. 1 B). Such traps are optimized for pests of economic significance by taking advantage of the chemical and visual cues associated with the resources insects search for, such as host plants for egg laying or mates for reproduction.Asian citrus psyllids (ACP) use multiple sensory modalities [vision, olfaction, contact chemoreception, gustation (taste), perception of auditory or vibrational stimuli] to locate host plants to feed on and reproduce. ACP are attracted to wavelengths perceived as yellow or lime-green in the human visual spectrum. UV wavelengths further increase ACP attraction to visual cues. An effective scent attractant blend can contribute to the attraction of ACP to visually attractive traps (Patt and Setamou 2010). After landing, ACP taste their potential food source and determine if the plant is suitable when they first insert their mouthparts into the plant. A specific blend of chemicals was identified that elicits a feeding response from ACP upon contact with an attractive substance (Lapointe et al. 2016). Currently available traps for monitoring ACP consist of flat, yellow-color panels (approx. 20 x 15 cm) coated with adhesive to ensnare encountering insects (Fig. 1A). In some cases, panels are also sold with proprietary blends of volatiles that purport to increase catch of ACP.
How does a better trap improve HLB management?
Trap devices for ACP are intended for: 1) detecting infestations in new areas; 2) triggering insecticide treatments to maintain psyllids below a threshold; and 3) timing insecticide sprays to have maximum impact on populations. Attractants for ACP can also be combined with a low-dosage killing agent into a single device that can be deployed for direct population reductions. Psyllids spread huanglongbing bacteria, and so reducing their numbers is a critical component of reducing impact of the disease.
Who is working on the Project?
Drs. Mamoudou Setamou (Texas A&M University), Steven Lapointe, David Hall, Joseph Patt (USDA-ARS-Fort Pierce, FL), Justin George, Xavier Martini, and Lukasz Stelinski (University of Florida), have collaborated extensively and also lead individual research efforts on identification of ACP attractants and development of practical ACP traps and lures.
What are the challenges?
The search for effective chemical attractants has yielded only weakly effective lures that enhance ACP adult orientation to visually attractive traps by only 2-2.5 fold. Currently available traps attract ACP from relatively short distances of only 6-30 feet, after ACP have already infested host plant foliage. The visual and olfactory cues of natural citrus foliage, particularly in a crop monoculture, far outcompete traps; they are simply much more attractive than foliage-mimicking traps. Thus, they are not very useful for drawing psyllids away from citrus.
In addition, such traps are not always reliable predictors of population outbreaks.
Efforts continue to refine chemical lures to improve their effectiveness under varying seasonal conditions and within diverse citrus genotypes grown in different regions. Furthermore, improvements continue on integrating several attractive cues (color, smell, and taste) with a toxicant to develop attract-&-kill stations that may alleviate the need for repeated insecticide spray applications for ACP management.
Citations:
Lapointe, S.L., George, J., and D. G. Hall. 2016. A phagostimulant blend for the Asian citrus psyllid. J. Chem. Ecol. 42: 941-951.Patt, J.M., Sétamou, M. 2010. Responses of the Asian citrus psyllid to volatiles emitted by the flushing shoots of its rutaceous host plants. Environ Entomol 39: 618–624.
Funding source: This project is funded by the USDA Multiagency Coordinated Funds.
-
Controlling psyllid gut cell death to prevent Huanglongbing
Research by Dr. Michelle Heck, USDA-Boyce Thomson Institute
Article written by Michelle Heck, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10, 2025.What is the technique?
Plant pathogens can have devastating effects on plant health and can severely limit food production in agricultural crops. In the case of the devastating disease, huanglongbing (HLB), the tiny Asian citrus psyllid is the vector that picks up the bacterial pathogen (Candidatus Liberibacter asiaticus - CLas) and transmits it to the next citrus tree during feeding. HLB is spread easily by psyllids. The focus of this research is determining if this transmission can be interrupted. Can the psyllid be prevented from picking up (acquiring) the pathogen when it feeds on an infected plant? And/or can the psyllid be prevented from transmitting the pathogen to a healthy plant?
How does targeting cell death help?
The first step in this research is determining how CLas affects the Asian citrus psyllid vector. Plant pathogens transmitted by insects are not always benign ‘tag alongs’ in the vector. They can have negative or positive effects on vector health and function. Also, vectors often respond to the pathogens with defense mechanisms, even if they are not targets of infection. Heck found that Asian citrus psyllids do respond to the CLas bacterium that causes HLB.
Heck’s group found that CLas causes cell death, or apoptosis, in the gut cells of the psyllid vector. Apoptosis, is a natural process of programmed cell death in multicellular organisms and its purpose is to get rid of unneeded or abnormal cells. Cells can initiate the proc
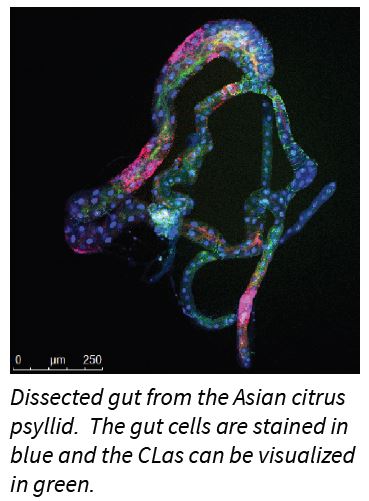 ess of cell death on their own, following stress or they can initiate the process when they receive signals from other cells. Though it’s a normal process, cell death can be shifted out of balance toward ‘too much or too little’ cell death and so affect the normal functions of the organism.
ess of cell death on their own, following stress or they can initiate the process when they receive signals from other cells. Though it’s a normal process, cell death can be shifted out of balance toward ‘too much or too little’ cell death and so affect the normal functions of the organism.Recent viral research suggests programmed cell death is a defensive mechanism that insects use to cope with viruses, which viruses in turn exploit to be successfully transmitted among hosts. The same may be taking place with the CLas - Asian citrus psyllid interaction. The psyllid’s gut cell death response may benefit the bacteria by allowing the bacteria to exit the gut and then enter the insects’ blood stream (hemolymph), ultimately reaching the insect’s mouthparts so that CLas can be passed on when the psyllid feeds on a new host plant.
Understanding how and why programmed cell death is triggered by CLas in the psyllid’s gut and whether this contributes to successful transmission among plants could have important implications for managing HLB. If the bacterial proteins that bind to the gut of the Asian citrus psyllid could be identified, then methods could be developed to impede the CLas bacteria’s ability to bind to gut cells, cause cell death and move into the insect’s blood stream.
Who is working on the Project?
Michelle Heck, a USDA researcher at the Boyce Thomson Institute, and her research team are exploring various ways to manage HLB by disrupting transmission by psyllids.
For more information see: Ghanim, M. et al. Morphological abnormalities and cell death in the Asian citrus psyllid (Diaphorina citri) midgut associated with Candidatus Liberibacter asiaticus. Sci. Rep. 6, 33418; doi: 10.1038/srep33418 (2016).
What are the challenges and opportunities?
Practical application of this new discovery requires finding specific proteins that bind to the gut and methods to alter or ‘silence’ them so that the bacteria can not bind to them, cause cell death and make their way into the blood stream of the insect. Development of this approach is estimated to take 3-5 years.
Funding source: This project is funded by USDA ARS, USDA NIFA, and the California Citrus Research Board.
-
Biological control of Asian citrus psyllid in California
Research by Dr. Mark Hoddle, University of California, Riverside
Article written by Mark Hoddle.
Revised July 10, 2025.What is biological control?
Biological control is the intentional use by humans of natural enemies, predators, parasitoids, and pathogens to reduce pest populations to less damaging levels. Classical biological control is the importation and release of a natural enemy species into an area where it is lacking. An example of classical biological control is the importation, release, and establishment in California of Tamarixia radiata, a parasitoid that attacks Asian citrus psyllid (ACP) nymphs, an invasive pest infesting citrus. The parasitoid was collected from Punjab Pakistan, which is part of the native range of ACP.
Punjab Pakistan was selected for foreign exploration because it has a very good (about 70%) climate match with major citrus production areas in California. Following release from quarantine in December 2011, Tamarixia established readily in California’s urban areas, it spread naturally into ACP-infested areas up to eight miles from the nearest release sites, and it has provided significant control of ACP.
Studies across numerous sites over several years indicate that ACP densities have declined in urban citrus by at least 70% and Tamarixia is one of the dominant natural enemies contributing to this mortality. Predatory hover fly larvae are important native natural enemies of immature ACP.
How does Tamarixia kill ACP?

Female Tamarixia lay eggs underneath the ACP nymph (A & B), the egg hatches, and the parasitoid larva consumes the inside of its host (C). The parasitoid larva pupates inside the shell of the ACP nymph (D & E), and after pupation is complete, the adult parasitoid chews a circular exit hole in the head region of the nymph and emerges (F). Tamarixia can also kill ACP nymphs by eating them, this is referred to as host feeding. To host feed, a female parasitoid uses her ovipositor to mutilate the nymph. This causes insect “blood” to leak from the host. The female parasitoid feeds on this liquid which provides protein for egg maturation. The trauma of being stabbed and fed on is sufficient to kill the ACP nymph (Figure 1).
Who is working on the Project?
The Joint Agency Biocontrol Taskforce for ACP biocontrol in California is comprised of team members representing UC Riverside (Mark Hoddle, Beth Grafton-Cardwell, Matt Daugherty, and Richard Stouthamer), CDFA (David Morgan, Victoria Hornbarker, and Mike Pitcairn), Citrus Research Board Scientists (Ruth Henderson, Raju Pandey, and Rick Dunn), Cal Poly Pomona (Anna Soper and Valerie Mellano), citrus growers (Jim Gorden and John Gless), pest control advisors (Joe Barcinas, Jim Davis, and Brett Chandler), and USDA-CPHST (Greg Simmons).
What are the challenges and opportunities?
Field studies in Southern California show that Argentine ants protect ACP nymphs from natural enemies in order to harvest the s
 ugary honeydew excreted by ACP nymphs. Ants kill Tamarixia that attempt to parasitize ACP nymphs. Thus, Argentine ant control is needed to maximize natural enemy impacts on ACP infestations. When ants are controlled, natural enemies (both Tamarixia and predators) exert substantial control of ACP. Novel approaches to ant control are being investigated and new monitoring and control technologies are being developed for potential use in citrus orchards (Figure 2).
ugary honeydew excreted by ACP nymphs. Ants kill Tamarixia that attempt to parasitize ACP nymphs. Thus, Argentine ant control is needed to maximize natural enemy impacts on ACP infestations. When ants are controlled, natural enemies (both Tamarixia and predators) exert substantial control of ACP. Novel approaches to ant control are being investigated and new monitoring and control technologies are being developed for potential use in citrus orchards (Figure 2).An additional challenge is that ACP natural enemies often lack food in citrus orchards, especially nectar and pollen, and the abse
 nce of these resources reduces their life span and how many pests they can kill. Flowering plants, like alyssum (Lobularia maritima) and buckwheat (Fagopyrum esculentum), attract adult hover flies. Experiments have demonstrated that the presence of flowering plants significantly increases mortality of ACP nymphs by hover fly larvae. More work is needed to determine the feasibility of growing flowering plants to attract hover flies in citrus orchards.
nce of these resources reduces their life span and how many pests they can kill. Flowering plants, like alyssum (Lobularia maritima) and buckwheat (Fagopyrum esculentum), attract adult hover flies. Experiments have demonstrated that the presence of flowering plants significantly increases mortality of ACP nymphs by hover fly larvae. More work is needed to determine the feasibility of growing flowering plants to attract hover flies in citrus orchards.Funding source: ACP biocontrol has been supported by funds from the Citrus Research Board, USDA-Multi Agency Coordination, USDA-Citrus Health Response Program, and UCR’s Office of Research and Economic Development.
-
Area-wide management of ACP to limit the spread of HLB in California
Research by Dr. Elizabeth Grafton-Cardwell, University of California, Davis
Article written by Sara Garcia-Figuera and Elizabeth Grafton-Cardwell.
Revised July 10, 2025Links to Dr. Grafton-Cardwell's podcast: Anchor | Apple | Spotify
What is the technique?
Area-wide pest management is the coordinated application of insecticides or some other control tactic on a large scale, beyond individual properties, with the goal of suppressing a pest population. In the case of huanglongbing (HLB), area-wide management aims to reduce the population of the insect vector, the Asian Citrus Psyllid (ACP), in order to limit the transmission of the bacterium that causes HLB.
In different areas of the world where HLB is present, like Brazil, Mexico or Florida, area-wide management of ACP is considered
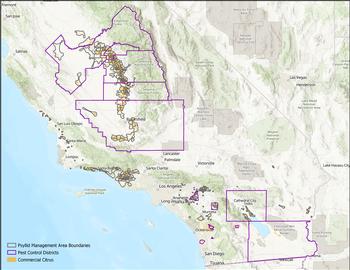 to be a fundamental pillar to reduce psyllids and control the spread of HLB. In California, area-wide management is voluntarily undertaken by growers in Psyllid Management Areas (PMAs) or mandated by Pest Control Districts (PCDs).
to be a fundamental pillar to reduce psyllids and control the spread of HLB. In California, area-wide management is voluntarily undertaken by growers in Psyllid Management Areas (PMAs) or mandated by Pest Control Districts (PCDs).PMAs are voluntary groups of 25-35 neighboring growers who coordinate insecticide applications against ACP over a short timeframe (2-3 weeks). Each PMA has a team leader who is responsible for contacting the rest of the growers when it is time to spray, following instructions from the regional grower liaisons and/or their ACP/HLB task force. PMAs have been generated for most citrus growing regions and can be viewed by clicking on the PMA layer of the
ACP Distribution and Management Map.PCDs are special districts instated by local growers to have the legal authority to control, eradicate, or respond to the effects of pests and diseases affecting a specific crop. For citrus, PCDs currently exist in portions of Fresno, Tulare, Kern, Riverside, San Diego and Imperial counties. In most of those counties, the PCD is responsible for indicating the timing of the area-wide ACP treatments. PCDs can be viewed by clicking on the PCD layer.
How does a coordinated effort improve HLB management?
By coordinating insecticide applications against ACP over a large area within a short timeframe, growers minimize movement of psyllids between neighboring fields or residential areas. The psyllids are very mobile and easily reinvade orchards once the insecticide breaks down. Treating over a wider area reduces reinvasion by adult psyllids, and the fewer the psyllids, the less likely they are to pick up and spread the disease.
In Southern California, where ACP is considered established and can no longer be eradicated, area-wide management is the best approach to minimize HLB transmission. Area-wide treatments are usually timed for the winter (Dec-Feb) to target the overwintering ACP population; in the spring (Feb-Mar) at the initiation of spring flush; and in the fall (Aug-Nov) to reduce the ACP population during the fall flush. Surveys of psyllids in California citrus orchards show that populations are highest in the fall and more aggressive choices and number of treatments are needed then.
In the San Joaquin Valley, where ACP is not so widely established, growers conduct voluntary coordinated treatments 800 m around the location where ACP is detected, in an attempt to locally-eradicate the population in that area. If psyllids are found on traps repeatedly in nearby 800 meter treatment areas, then growers work together to simultaneously treat a much larger area. In all areas of the state, when 90% or more growers participate in a coordinated treatment in a short time frame, the California Department of Food and Agriculture (CDFA) applies insecticides to residential citrus within 400 meters of that treatment, to reduce ACP reinfestation.
Who is working on the Project?
This grower-led initiative is based on neighbors helping neighbors. However, citrus growers are not alone. The Citrus Pest and Disease Prevention Program (CPDPP) has appointed grower liaisons to inform growers and pest control advisors about ACP detections and help them determine when it is time to spray. The design of the PMAs was a collaborative effort between Rick Dunn of the Citrus Research Board, citrus growers, the University of California and CDFA liaisons. The insecticide recommendations and treatment timings were developed by Dr. Beth Grafton-Cardwell. Specific ACP/HLB task forces have been created in most of the counties, and the PCDs can provide structure, recommendations, complimentary activities such as tree removal, and sometimes funding depending on their budgets. The California Citrus Mutual provides funding in some areas of the state for residential tree removal.
What are the challenges and opportunities?
The success of the area-wide management of ACP depends on the willingness to coordinate among citrus growers. Studies conducted in Florida by Dr. Ariel Singerman showed that the Citrus Health Management Areas (CHMAs) were not always successful because of trust issues among neighbors and lack of flexibility to spray when it is required. However, it is vital to overcome these obstacles and coordinate efforts to keep ACP populations to the minimum, even before HLB is detected, in order to limit the impact of the epidemic in California.
-
Using interference RNA to manage Asian citrus psyllids
Research by Drs. Lukasz Stelinski and Nabil Killiny, University of Florida
Article written by Lukasz Stelinski, Nabil Killiny, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10,2025.What is the technique?
Insecticides are one of the main tools used to manage the Asian citrus psyllid. Their overuse has led to the evolution of insecticide resistance in some populations of Asian citrus psyllids, rendering them less susceptible to insecticide treatment. This is particularly concerning for the neonicotinoid class of insecticides, since these are the main tools currently being used to protect young trees from psyllids carrying CLas, the bacteria that causes huanglongbing. If this problem is not properly managed, insecticides could become less effective in killing psyllids in the field.
Researchers are developing a way to render field populations of psyllids more susceptible to insecticides, using a technique called RNA interference, or RNAi. RNAi can be used to precisely target and shut down important genes in Asian citrus psyllids to manage the insect’s response to insecticides.
Using RNAi to prevent insecticide degradation
Insecticide resistance to the group of pesticides called neonicotinoids happens when insects increase certain enzymes (encoded by CYP4 genes) that break down the pesticide.
RNAi can be used to shut down the making of gene products, like the enzymes that degrade insecticides, and so make insecticides more effective against Asian citrus psyllids and so reverse insecticide resistance.

Silencing the above-mentioned CYP4 genes, by feeding newly emerged Asian citrus psyllids with dsRNA (double stranded RNA) molecules, inhibits expression of the Cyp4 gene rendered them more susceptible to insecticides. In the laboratory, they were able to completely reverse insecticide resistance of psyllids collected from farms whose psyllids were showing resistance (Figure 1).
Killiny’s group also used RNAi to combat Asian citrus psyllids directly. First, they identified several key genes important in the life history of Asian citrus psyllid, such as those responsible for normally functioning wings and flight muscles. They developed specific dsRNAs, which when fed to immature psyllids, resulted in adults that emerged with malformed wings and were incapable of flight (see above). Thus the resulting psyllids cannot transmit the bacteria causing huanglongbing.
Who is working on the Project?
Lukasz Stelinski, an associate professor at the University of Florida, is leading efforts on insecticide resistance research for Asian citrus psyllid in Florida. Nabil Killiny, an assistant professor with University of Florida, is leading the efforts to develop RNAi-based management tools for Asian citrus psyllid.
What are the challenges and opportunities?
An important challenge for the practical use of dsRNA for pest control is to figure a way to get the dsRNAs into the insect in the field. Killiny and others are working on inserting dsRNAs into citrus plants, so the Asian citrus psyllid would ingest them during feeding. Delivery of dsRNA through transgenic plants has been achieved with other insect pests and thus should be possible to do for Asian citrus psyllid.
Another potentially feasible way to deliver dsRNA is to incorporate dsRNA into transgenic bacteria that are not harmful to humans and then spraying the transgenic bacteria onto citrus trees. However, a practical limitation of the RNAi approach is that large quantities of dsRNA are needed and are expensive to produce. Also, research is needed to develop formulations that prevent breakdown of dsRNA under field conditions.
Funding source: This project is funded by the Citrus Research and Development Foundation.
-
Altering the Asian citrus psyllid's beneficial bacteria to stop HLB spread
Research by Dr. Kirsten Pelz-Stelinski, University of Florida
Article written by Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised June 30, 2025.What is the technique?
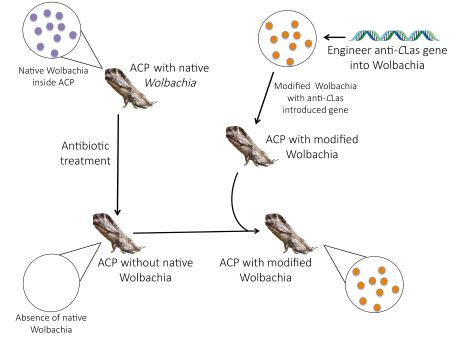
The insect vector, Asian citrus psyllid, spreads the CLas bacterium that causes HLB when it feeds. So, if we can find ways to interrupt this transmission of CLas by the psyllid, we may be able to stop the disease. One way to stop transmission is to genetically alter the naturally occurring organisms (endosymbionts) in the psyllid bodies and use them as a vehicle to bring anti-CLas compounds into the psyllid. Then, we can replace the wild populations of psyllids with psyllids unable to transmit CLas.
How can manipulating symbionts reduce psyllid populations?
Cells of Asian citrus psyllids harbor at least three bacteria inside their bodies, including one called Wolbachia. These bacteria are c
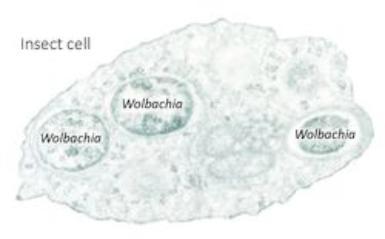 alled endosymbionts, because they live inside the psyllid (endo) (below) and they help the psyllid live and grow (symbionts). They seem to be unaffected by the presence of the HLB pathogen, CLas. This makes Wolbachia a candidate for modification (Figure 1).
alled endosymbionts, because they live inside the psyllid (endo) (below) and they help the psyllid live and grow (symbionts). They seem to be unaffected by the presence of the HLB pathogen, CLas. This makes Wolbachia a candidate for modification (Figure 1). If Wolbachia can be genetically modified so that it carries anti-Clas compounds and then introduced into Asian citrus psyllids (above right), then it can be used as a vehicle for delivery of those compounds into the psyllid. The key to this strategy is to find a symbiont that has a harmonious relationship with the host psyllid and also can be genetically manipulated. If successful, the symbiont is converted into a biocontrol agent that causes the death of CLas. If the research program is successful, the plan is to release Asian citrus psyllids that contain Wolbachia that are making anti-CLas compounds that then interbreed with and displace the disease-transmitting psyllid populations, helping to stop HLB.
Disease management applications
This approach has been used in mosquitos, where a population of the mosquito was made incapable of transmitting the Dengue virus, following its infection with a genetically altered Wolbachia bacterium. Mosquitos don’t normally have Wolbachia in their bodies, and if mosquitos are infected with Wolbachia and released over a number of weeks, they mate with the wild mosquitoes and gradually the percentage of mosquitoes with Wolbachia increases. Mosquitoes with the Wolbachia are less able to transmit viruses to people and the outbreaks of Dengue are reduced (Figure 2).
Who is working on the Project?
Kirsten Pelz-Stelinski, an associate professor with the University of Florida, is leading the research efforts on exploiting Wolbachia endosymbionts within Asian citrus psyllids to disrupt the transmission of HLB. Collaborative research with other groups for development of large-scale cultures of such psyllids for future release as a management tool is ongoing.
What are the challenges and opportunities?
One of the biggest challenges in this approach is to keep the Wolbachia healthy and competitive after its genetic information has been altered to include the anti-CLas compounds, so that it can compete with the Wolbachia in the wild psyllid population. Understanding the mechanisms of the transmission process and then translating that understanding into mass culturing of psyllids for field release presents significant logistical and regulatory hurdles. However, if successful, the psyllids could be released in existing citrus orchards and reduce transmission of HLB, helping to save the citrus industry.
Funding source: This project is funded by the USDA-NIFA.
-
Using tristeza virus to provide citrus with anti-microbial or insecticidal
Research by Drs. Bill Dawson and Kirsten Pelz-Stelinski, University of Florida
Article written by Bill Dawson, Kirsten Pelz-Stelinski, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised June 27, 2025.What is the technique?
A novel way to kill Asian citrus psyllids is to insert into the citrus plant insect-derived or plant-derived peptides. These are short portions of protein that have insecticidal (kill the insect) or antimicrobial (kill CLas) activity. An example of a plant-based insecticidal peptide is lectins, that act against phloem-feeding insects. Lectins affect receptors in the insect gut, causing lesions that prevent the insect from feeding, and so it dies. Antimicrobial peptides affect the internal communities of endosymbiotic (helpful) bacteria in psyllids, reducing psyllid fitness. Antimicrobial peptides also have the potential to kill CLas bacteria directly, if selective antimicrobials can be found, engineered into plants, and delivered to the psyllid.
Using viruses as delivery tools
Antimicrobial peptides could be inserted into citrus via genetic engineering, but this approach requires replanting of existing citrus trees and the process would need to be repeated for each variety of citrus. An alternative method to deliver peptides, is to engineer a mild strain of the citrus tristeza virus (CTV) that, in itself is not damaging to citrus, but it can express insecticidal or antimicrobial peptides. This altered CTV would act as a carrier to inoculate trees and so would not require the trees to be replanted – creating an approach that is much faster than engineering the plant.
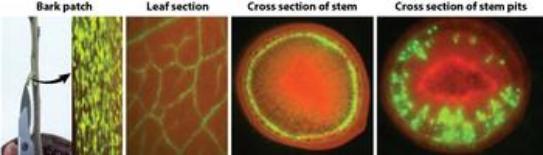
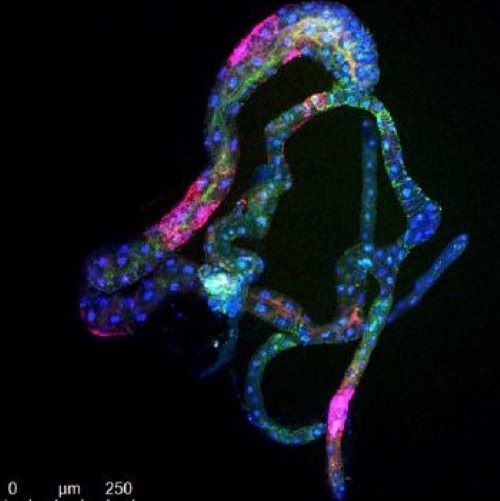
In order to show visually where the virus ends up in the tree, Dawson created a virus that makes a visual green fluorescent protein (GFP). CTV was shown to produce GFP (green color) to very high levels (below) throughout the tree. Recently, Dawson and his collaborators have used the vector to produce a wide range of antimicrobial peptides to control HLB.
Who is working on the Project?
Bill Dawson at the University of Florida, has pioneered research on development of CTV vectors to transport foreign genes within plants. Through his laboratories’ research, CTV vectors have been developed to express foreign genes throughout citrus trees, acting like ‘vehicles’ to deliver antimicrobials into the tree. Dr. Kirsten Pelz-Stelinski, also of the University of Florida is collaborating in learning how the insecticidal peptides affect psyllids.
What are the challenges and opportunities?
Citrus budwood, containing a mild strain of CTV that makes insecticidal or antibacterial peptides specific for psyllids, could be grafted onto nursery trees. Or, the CTV budwood could be graft-inoculated onto existing trees, negating the need for tree removal and replanting, and would not require developing new technologies for different varieties. Because making the peptide is not a permanent ability of the virus, it eliminates the possibility of the trait escaping in pollen. The major limiting factors in developing this technology are finding effective antimicrobial and insecticidal peptides to incorporate into the CTV and obtaining approval for their use from the FDA and EPA. To this end, on May 10, 2018 USDA APHIS released a draft Environmental Impact Statement for a 45 d period for public comment (https://www.regulations.gov/docket?D=APHIS-2017-0018). This document describes the environmental impacts that might result from the environmental release throughout Florida of genetically engineered Citrus tristeza virus. They also included an analysis of the plant pest risks associated with using this engineered virus as a biological control agent to help manage HLB.
Citation: Dawson, W.O., Bar-Joseph, M., Garnsey, and Moreno, P. 2015. Citrus Tristeza Virus: Making an Ally from an Enemy. Annual Review of Phytopathology 53: 137-155. http://www.annualreviews.org/doi/full/10.1146/annurev-phyto-080614-120012
Funding source: This project is funded by USDA-NIFA.
-
A new, Bt toxin-based strategy for suppression of the Asian citrus psyllid vector of HLB
Dr. Bryony Bonning, University of Florida
Article written by Bryony Bonning, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10, 2025.What is the technique?
When the Asian citrus psyllid feeds, it can inoculate the host plant with the CLas bacterium that causes HLB or citrus greening, causing severe damage to the plant. A multi-pronged approach is required for management of this complex citrus disease. One approach to suppress the numbers of psyllid vectors is to use naturally occurring insecticidal toxins that are produced by a soil-borne bacterium, Bacillus thuringiensis (Bt). These toxins are harmless to humans, but are toxic to specific insect species.
How can Bt toxins be used to reduce psyllid populations?
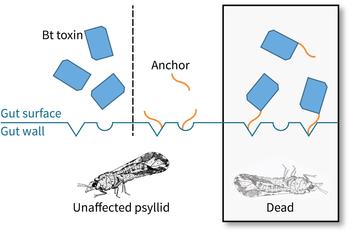
When ingested, Bt toxins bind to the gut wall of an insect and create a hole. This damage stops the insect from feeding and ultimately kills it. Bt toxins have been used widely in organic agriculture and more recently in insect-resistant transgenic cotton and corn in the United States. A challenge for use of this strategy against the Asian citrus psyllid, however, is that Bt toxins don’t typically work very well against insects that feed on plant sap, because the toxins don’t bind well to the gut of these types of insects. Fortunately, a new approach provides the toxin with an artificial anchor, so that the toxin can bind to the psyllid gut and exert its effect.
Disease management applications
Once we have surveye
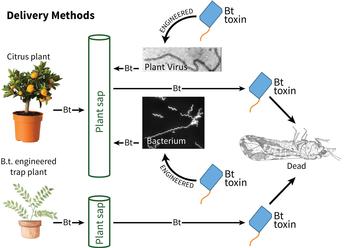 d for and developed Bt toxins with anchors that kill Asian citrus psyllids, a system for appropriate delivery of the toxins is needed. To be ingested as the psyllid feeds, the toxins have to be located in plant sap. There are several options to accomplish this. One approach is to use microbes or viruses that naturally reside in plant sap, as toxin delivery vehicles. For example, an innocuous plant virus or bacterium that resides in the sap, can be engineered to produce toxin. Alternatively, plants could be engineered to produce toxin in the sap. This latter strategy could be used either for citrus or for a so-called “trap plant” that is more attractive to psyllids than citrus. In this approach, psyllids would be enticed to the trap plant that produces Bt toxin and killed, reducing
d for and developed Bt toxins with anchors that kill Asian citrus psyllids, a system for appropriate delivery of the toxins is needed. To be ingested as the psyllid feeds, the toxins have to be located in plant sap. There are several options to accomplish this. One approach is to use microbes or viruses that naturally reside in plant sap, as toxin delivery vehicles. For example, an innocuous plant virus or bacterium that resides in the sap, can be engineered to produce toxin. Alternatively, plants could be engineered to produce toxin in the sap. This latter strategy could be used either for citrus or for a so-called “trap plant” that is more attractive to psyllids than citrus. In this approach, psyllids would be enticed to the trap plant that produces Bt toxin and killed, reducing
the number of insects that land on citrus trees.Who is working on this project?
Bryony Bonning, Professor at the University of Florida, is leading a team of 11 primary investigators, drawn from USDA ARS in Maryland (Michael Blackburn), University of California at Davis (Karen Jetter) and at Riverside (Caroline Roper, James Borneman, Elizabeth Grafton-Cardwell), Texas A&M (Mamoudou Setamou) and University of Florida (James Keesling, William Dawson, Lukasz Stelinski, Vladimir Orbovic).
What are the challenges and opportunities?
A primary challenge for the project is the appropriate delivery of sufficient toxin from the sap to kill the psyllid, as each potential delivery system has its own set of limitations. Once an appropriate delivery system has been identified, regulatory hurdles will have to be overcome for adoption of this strategy by growers. The main opportunity provided by this research is the use of a unique psyllid management approach that can be integrated with other approaches for management of HLB, to the benefit of the U.S. citrus industry.
Funding source: The Citrus Research & Development Foundation funded the initial work. The current funding is from the USDA Citrus Disease and Extension (CDRE) NIFA.
-
Using insect viruses to combat the Asian citrus psyllid
Research by Dr. Bryce Falk, University of California, Davis
Article written by Bryce Falk, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10, 2025.Insects have viruses just like people have viruses. In fact, viruses are the most abundant microbes on our planet. Most viruses are discovered only when they cause disease in their hosts. An example is the human immunodeficiency virus (HIV), the pathogen that causes AIDS. This virus is not new, but has existed for thousands of years in its natural host, most likely non-human primates, where it did not cause disease. It remained undiscovered until it shifted to humans and caused disease. Bryce Falk’s research is focused on finding and identifying viruses in the Asian citrus psyllid (Diaphorina citri; D. citri). These viruses, or genetically altered versions of them, could be potential tools to combat the Asian citrus psyllid to limit spread of the bacterium (Candidatus Liberibacter asiaticus; CLas) that causes huanglongbing (HLB) disease of citrus.
What is the technique?
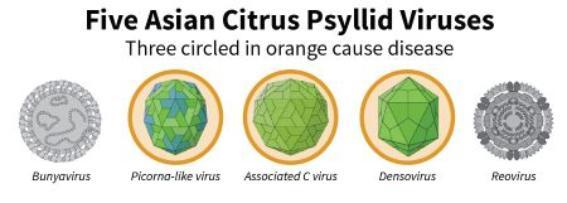
Falk’s lab has collected viruses (as ribonucleic acid, RNA) from Asian citrus psyllids all around the world - including China, Pakistan, Taiwan, Brazil, Florida, Puerto Rico, Texas, Hawaii, Arizona and California. His lab collects RNA, rather than DNA, because most insect viruses have RNA as their genetic material and even those viruses with DNA genomes must make RNA during infection. The RNA from the virus is non-infectious and can be safely imported into the United States for analysis. RNA can also be used for sequencing of the genes in order to devise strategies to use PCR-based (polymerase chain reaction) techniques to find new viruses. Falks’s lab has collected 6 viruses from Asian citrus psyllids, and are evaluating various strategies to use one or more in the battle against HLB. The first strategy is to use the virus, as is, to sicken? psyllids and attempt to disrupt CLas transmission. His lab is studying the viruses separately and in combinations to assess whether they work better affecting CLas when alone or co-infecting the psyllid. The second strategy is to genetically modify several of the viruses so that they will induce traits in the psyllid that make it less likely to transmit CLas. These viruses include a picorna-like virus, an associated C virus, and a denso virus. Currently they are putting these viruses into cell culture systems to understand their genetics and how they work. Similar efforts with viruses are underway for mosquitoes that spread diseases, such as malaria. Thus, this project is timely and takes advantage of new opportunities to target the Asian citrus psyllid. Understanding the psyllid genes that interact with the viruses could provide a clue as to how psyllids resist viruses, which then could against them by altering the genetics of the insect or the virus (Figure 1).
 Who is working on the Project?
Who is working on the Project?Bryce Falk, Professor of Plant Pathology at UC Davis, and his laboratory are studying the viruses, developing insect cell culture lines to grow the viruses, and determining if they could be used to reduce psyllid populations or negatively impact psyllid transmission of CLas. This work is done within the UC Davis biosafety 3P Contained Research Facility (crf.ucdavis.edu) where they maintain four populations of the Asian citrus psyllid collected from various parts of the world. They maintain the viruses on cell cultures made up of other insects, such as fruit flies, moths or plant insects, and in D. citri.
What are the challenges and opportunities?
Much research needs to be done to understand the genetic make-up of the viruses and how they act in the psyllid. If a virus naturally competes with the HLB bacterium in the psyllid or could be modified to compete with the bacterium, or in some other way reduce the psyllid’s ability to transmit the bacterium, then it could be used to slow the spread of HLB.
Funding source: This project is funded by the USDA-NIFA and the USDA-APHIS-PPQ.
-
Using peptides as a preventive approach to target the psyllid and the pathogen
Research by Dr. Robert Shatters, USDA Horticultural Research Laboratory, Fort Pierce, FL
Article written by Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10, 2025.Peptides are short chains that come from naturally occurring proteins. Most organisms produce a special group of peptides that act as a first line of defense against bacteria, interfering with their ability to transmit disease. USDA-ARS researcher, Dr. Robert Shatters, along with others, is looking at the possible use of antimicrobial peptides to stop the spread of the CLas bacterium that causes huanglongbing (HLB) disease of citrus, in concert with peptides that affect the Asian citrus psyllid’s ability to acquire and transmit the pathogenic bacterium. The quickest approach to use these peptides would be in topical applications to the leaves of citrus trees, while a long term approach would be to engineer citrus with the genes to produce the peptides.
What is the technique?
Dr. Shatters and colleagues are pursuing three different application strategies to deliver a combination of peptides with the combined effect of killing the psyllid, or blocking the psyllid from acquiring and transmitting the bacterium, and/or directly killing the bacteri
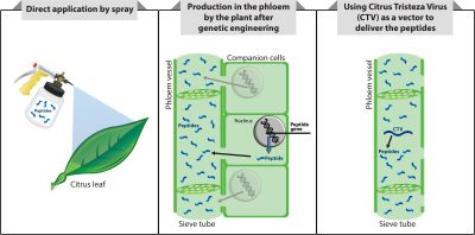 um. The first peptide deliver strategy would be a spray application of peptides directly on the plant. This approach would be the quickest solution for growers; however, it would require repeated applications. A second strategy involves engineering citrus to produce the peptides directly in the phloem, the part of the plant where the bacterium lives. This approach avoids the necessity of having to repeatedly applying the peptides. However, it would work only on new plantings of genetically engineered citrus trees, not on existing trees and the regulatory pathway to permit its commercial use would be longer than direct applications. A third approach , conducted collaboratively with Dr. Bill Dawson, at the University of Florida, involves inserting genes for the peptides into Citrus tristeza virus (CTV). The virus would be transferred to the citrus tree by grafting CTV-infected tissue onto the tree. The engineered CTV would live in the phloem and produce peptides that would circulate through the phloem. This strategy will require only a single application. It could be used on existing plantings and would require a longer regulatory process than the sprayable peptides.
um. The first peptide deliver strategy would be a spray application of peptides directly on the plant. This approach would be the quickest solution for growers; however, it would require repeated applications. A second strategy involves engineering citrus to produce the peptides directly in the phloem, the part of the plant where the bacterium lives. This approach avoids the necessity of having to repeatedly applying the peptides. However, it would work only on new plantings of genetically engineered citrus trees, not on existing trees and the regulatory pathway to permit its commercial use would be longer than direct applications. A third approach , conducted collaboratively with Dr. Bill Dawson, at the University of Florida, involves inserting genes for the peptides into Citrus tristeza virus (CTV). The virus would be transferred to the citrus tree by grafting CTV-infected tissue onto the tree. The engineered CTV would live in the phloem and produce peptides that would circulate through the phloem. This strategy will require only a single application. It could be used on existing plantings and would require a longer regulatory process than the sprayable peptides.Who is working on the project?
Dr. Robert Shatters, a Research Molecular Biologist at the USDA Horticultural Research Laboratory in Ft. Pierce FL, and his laboratory have demonstrated that by combining antimicrobial peptides with acquisition/transmission blocking peptides, up to 90% of the psyllids were killed in lab experiments and pathogenic bacteria were completely blocked from being acquired by the psyllids that survived. This group is now moving this work to greenhouse and research field to determine how well these peptides function under whole-plant and field conditions. This work is being conducted collaboratively with other scientists. Dr. Michelle Cilia, USDA-ARS in Ithaca NY, is identifying the specific protein interactions involved with the gut binding peptides; Dr. Ed Stover, USDA-ARS, Fort Pierce FL, is working to produce transgenic citrus expressing these peptides; and Dr. Bill Dawson, University of Florida, is investigating use of the CTV virus to produce these peptides within infected citrus.
What are the challenges and opportunities?
Using peptides to reduce disease is not a new technique, as they are currently being used in human health to attempt to combat damage from cardiovascular disease.
Because citrus is constantly being re-infected with the CLas bacterium, this approach will most likely serve as a preventative. However, in California, where psyllid and tree infection rates are very low, it is possible this strategy could be a cure. Experimentally, bacterial populations were reduced by peptides in citrus leaves by >99% after five days (based on detectable bacterial DNA). Thus, over time, treatment with peptides could result in a cure, if peptides were shown to reach throughout the plant, including the roots.
In order for these approaches to be effective in reducing HLB in citrus, a number of parts to this research puzzle need to fall into place. First, the peptides have to be effective against the bacterium. Second, the delivery of the peptides into the psyllid needs to be effective and economically viable. Third, to be useful for growers, the regulatory pathway must be established that will permit these tools to be deployed in the field. It is encouraging that commercial peptide-based topical sprays were developed and used by growers in the 1990’s to induce plant defenses. And, since then, more economical methods of producing peptides have been achieved. If these three hurdles can be overcome, peptides may become a useful tool to combat HLB.
-
Growing citrus under enclosures
Research by Dr. Rhuanito Soranz Ferrarezi, University of Florida
Article written by Rhuanito Soranz Ferrarezi, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10, 2025.What is the technique?
Citrus production under protected environments reduces huanglongbing (HLB) disease incidence and damage by excluding the Asian citrus psyllid (ACP) vector from access to citrus trees by surrounding them with a screen barrier. In addition to protecting citrus from the disease, it greatly reduces insecticidal sprays applied to control psyllids. This reduces costs and selection for insecticide resistance in the psyllid.
How does it improve HLB management?

Completely enclosed screenhouses physically exclude the ACP and thus prevent inoculation of the plants with the CLas bacteria that causes HLB. A screened enclosure in Florida fully protected young grapefruit (Citrus × paradisi) trees from eggs, nymphs and adult ACP and no trees tested positive for CLas inside the enclosure after two years of monitoring. In contrast 100% of surveyed trees in the nearby open-air plots tested positive for CLas during the same period. Thus, the use of screenhouses offered a substantial level of protection against the establishment of HLB, compared to management programs founded solely upon protecting the citrus from ACP using insecticidal sprays.
Who is working on the Project?
The citrus under protective screen system was developed at the University of Florida/Institute of Food and Agricultural Sciences (UF/IFAS) Indian River Research and Education Center in Fort Pierce, FL and has been tested at the UF/IFAS Citrus Research and Education Center in Lake Alfred, FL. The economics of citrus under protective screens (CUPS) is being determined. To date, there are 50 acres of commercial CUPS with three growers in Florida, and at least 150 more acres are planned (Eduardo Pines and Steven Callaham, personal communication).
Drs. Rhuanito Ferrarezi, Arnold Schumann, Ariel Singerman, Jawwad Qureshi, Philippe Rolshausen (UC Davis), Andrew Krajewski (International Citrus Technologies, Australia), Elizabeth Grafton-Cardwell, Stephen Futch, Chris Oswalt and Garima Kakkar are currently part of this research.
Dr. Ferrarezi is with the University of Florida Horticultural Sciences Department He is a professor of citrus horticulture and is located at the Indian River Research and Education Center in Fort Pierce, FL. All other researchers with the exceptions of Grafton-Cardwell (UC Riverside), Krajewski (International Citrus Technologies, Australia), and Rolshausen (UC Davis) are at the University of Florida.
What are the challenges and opportunities?
Researchers are still answering many questions about the technology, such as the most suitable citrus varieties, the installation and maintenance costs, the economics and level of fruit production, the level of labor needed, pest and disease control, resistance to extreme weather conditions such as frost, tropical storms and hurricanes, irrigation and fertilization, canopy management and other technical aspects. The Florida CUPS group recently received a USDA-NIFA-CDRE grant to address a number of these topics.
Ferrarezi RS, Wright AL, Boman BJ, Schumann AW, Gmitter FG, Grosser JW. 2017. Protected fresh grapefruit cultivation systems: Antipsyllid screen effects on environmental variables inside enclosures. HortTechnology 27(5): 675-681.
http://dx.doi.org/10.21273/HORTTECH03790-17Ferrarezi RS, Wright AL, Boman BJ, Schumann AW, Gmitter FG, Grosser JW. 2017. Protected fresh grapefruit cultivation systems: Antipsyllid screen effects on plant growth and leaf transpiration, vapor pressure de?cit, and nutrition.
HortTechnology 27(5): 666-674. http://dx.doi.org/10.21273/HORTTECH03789-17
Funding source: This project is funded by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2018-70016-27387. -
SP: Virus-induced gene selencing (VIGS) using insect-specific viruses to manipulate psyllids as a strategy to help control citrus greening/HLB
Research by: Diogo M. Galdeano, Curtis R. Carson, Emilyn E. Matsumura, Tanvi Rawat, William Ingram, Jun Jiang, Bryce W. Falk, and Yen-Wen Kuo
Article written by: Yen-Wen Kuo
Article edited by: Bryce W. Falk, Diogo M. Galdeano, Ed Stover, Peggy G. Lemaux
Revised July 15, 2025.
What is the technique?RNA interference (RNAi) is a biological mechanism where small RNAs bind to RNA and prevent the RNA translation process or where they degrade long RNAs with a specific targeted sequence, resulting in a change in gene expression and related metabolism. This mechanism was found to be essential not only for regulating gene expression in plants and animals, but also for host defense mechanisms. In this project, we are developing non-pathogenic virus-based RNAi induction to interfere with Asian citrus psyllid (Diaphorina citri) infection - with the goal of suppressing HLB. Applications, such as sprays of double-strand RNAs to induce RNAi in citrus, are expensive and inefficient. For sustained and effective solutions to block or suppress Candidatus Liberibacter asiaticus (CLas) in D. citri, we are developing a virus-based system using viral endosymbionts of D. citri. These endosymbionts are viruses that live in close physical association with plant cells and are in a situation in which both organisms benefit.
How is that procedure involved in the research?With improved DNA/RNA sequencing, scientists have discovered a diversity of viruses in insects, most of which are passed through generations, but induce no disease. Our group has identified multiple D. citri-specific viruses. The main goal for our current project is to complement HLB management efforts through manipulating the balance and/or interactions between D. citri endosymbionts, which are essential for insect metabolism and immune responses, to block acquisition and/or transmission of CLas (Figure 1). We are constructing infectious clones of four D. citri-specific viruses and evaluating effects of those viruses on D. citri populations, collected from California, Uruguay, and Taiwan. The variety of different types of viruses could be helpful for future applications in different D. citri populations and for different purposes. In addition to constructing viral infectious clones of the D. citri-specific viruses, we are studying the biology of these viruses to gain a fundamental understanding of this complex system to ensure that we use optimal approaches.
Who is working on the project?Researchers on this project include Diogo M. Galdeano, Curtis R. Carson, Tanvi Rawat, William Ingram, Bryce W. Falk, and Yen-Wen Kuo at the University of California, Davis, Emilyn E. Matsumura at the Wageningen University & Research in the Netherlands, and Jun Jiang, currently at Shandong Agricultural University, China.
What are the challenges?To develop a comprehensive and thorough insect-specific virus (ISV)-based management strategy against HLB, we have simultaneously studied the D. citri genome that provides an important foundation for future D. citri and citrus greening studies. The biology studies of D. citri and its viral endosymbionts will help us choose the best potential target sequences to achieve the desired effects on Clas transmission. The breadth of our D. citri genetic resources, D. citri viruses in culture, and the depth of data generated so far make us uniquely able to use viral endosymbionts as future tools for citrus greening biocontrol strategies.
Figure 1. Main concept for Diaphorina citri-specific virus applications. The D. citri-specific viruses that were identified infecting D. citri (Asian citrus psyllid) in different geographical populations are being developed into viral vectors. Those viruses can vertically transmit to the progenies and spread into D. citri populations, to target D. citri, Candidatus Liberibacter asiaticus (CLas, causal agent of Huanglongbing), or other endosymbionts in D. citri. The targets will be designed to block or suppress CLas transmission or to be lethal to D. citri. Different viruses will be used for different D. citri populations based on our results from the biology assays
Funding source: United States Department of Agriculture-National Institute of Food and Agriculture (USDA-NIFA), Emergency Citrus Disease Research and Extension (ECDRE) Program
-
Reducing Asian citrus psyllid and disease incidence with reflective mulches
Research by Drs. Phil Stansly and Scott Croxton, University of Florida
Article written by Phil Stansly, Scott Croxton, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised August 5, 2025.What is the technique?
Reflective mulches are used by vegetable growers to repel plant-sucking insects such as aphids, whiteflies, and thrips that often colonize young crop plants and cause serious yield reductions due to direct feeding injury and disease transmission. These metalized mulches reflect UV light and are thus thought to disorient day flying insects that use the positioning of the sun as an orientation cue for navigation. Simple use of aluminum foil as a repellent for aphids was tested in vegetable crops as early as the 1950s. Since these early days of testing, much more weather resistant reflective mulches have been applied as a means of pest control in many crops, particularly vegetables, and have targeted several pests, often improving yields as a result of reduced pest densities. Such mulches are sometimes used in concert with targeted application of systemic insecticides and can protect plants from infection by insect-transmitted viruses.
The Asian citrus psyllid (ACP) vector of the bacteria that cause citrus greening disease also known as huanglongbing (HLB), appeared to be an ideal candidate for susceptibility to management with reflective mulches. ACP is a day flying insect and primarily relies on vision to orient to their host plants for feeding and egg laying. As a crop, citrus is also a good candidate for treatment with ground cover mulches, since the crop often requires careful management of weed and irrigation for optimal growth. Therefore, researchers investigated the effects of applying ground cover reflective mulches in young citrus trees on the level of ACP infestation, HLB infection, tree growth, and weed incidence.
How does it improve HLB management?
Metalized mulches were found to reduce densities of ACP flying into treated plots over a 7-month sampling period as compared with control plots with bare ground or those that were treated with white-painted mulch covers. Furthermore, repelling ACP with
 the metalized mulch reduced the incidence of HLB infection by 45 % in the first year of the investigation and by 20% the following year. Furthermore, metalized mulch treatment in concert with drip irrigation approximately doubled growth of young trees as compared with those grown in bare ground with the standard micro-sprinkler irrigation practices used by growers. Weed growth was significantly reduced, as well as water use for irrigation as compared with the grower standard treatments and bare ground controls.
the metalized mulch reduced the incidence of HLB infection by 45 % in the first year of the investigation and by 20% the following year. Furthermore, metalized mulch treatment in concert with drip irrigation approximately doubled growth of young trees as compared with those grown in bare ground with the standard micro-sprinkler irrigation practices used by growers. Weed growth was significantly reduced, as well as water use for irrigation as compared with the grower standard treatments and bare ground controls.This initial proof of concept experiment consisted of 10 tree small plots of newly planted citrus seedlings replicated four times. In follow-up experiments with 100 tree plots rep
 licated 5 times, protection of trees from HLB infection with metalized mulch was even more effective, particularly when incorporating a well-timed soil drench of a neonicotinoid insecticide. Furthermore, fruit yield and juice volume were both increased by using metalized refle
licated 5 times, protection of trees from HLB infection with metalized mulch was even more effective, particularly when incorporating a well-timed soil drench of a neonicotinoid insecticide. Furthermore, fruit yield and juice volume were both increased by using metalized refle ctive mulch treatment for ACP and HLB management as compared with bare ground controls at 143 weeks after treatments were applied. The researchers suggest that scaling up treatment acreage may further improve protection of young trees from HLB, as well as further improve growth of citrus and fruit yield in areas where HLB is endemic.
ctive mulch treatment for ACP and HLB management as compared with bare ground controls at 143 weeks after treatments were applied. The researchers suggest that scaling up treatment acreage may further improve protection of young trees from HLB, as well as further improve growth of citrus and fruit yield in areas where HLB is endemic.Who is working on the Project?
Phil Stansly and Scott Croxton conceived, designed, and carried out this research. Stansly is with the University of Florida’s Institute of Food and Agricultural Sciences. He is a professor of entomology and is located at the Southwest Florida Research and Education Center in Immokalee, FL. Croxton is a former University of Florida graduate student and currently with Nichino America.
What are the challenges and opportunities?
Use of metalized reflective mulches requires an initial investment in mulch, bedding equipment, and drip irrigation, which has not been standard practice in large-scale citriculture in Florida. Also, it is not a stand alone treatment that will completely eliminate psyllid population without integration with other pest management tools. However, the integration of reflective mulch and drip irrigation with use of well-timed systemic insecticide treatment has been shown to deter ACP infestation, reduce HLB incidence and severity, and increase tree growth and early yield. Initial economic analysis of the small-plot trials suggest a profit benefit of the approaches given the significant improvement in yield as compared with planting citrus seedlings with bare ground. Current opportunities being investigated include improving the shape of citrus beds planted with mulch, bedding equipment, irrigation plans, and mulch longevity. Further economic analysis of larger scale applications is also underway.
Croxton SD, Stansly PA. 2013. Metalized polyethylene mulch to repel Asian citrus psyllid, slow the spread of huanglongbing and improve growth of new citrus plantings. Pest Management Science. 70: 318-323. http://onlinelibrary.wiley.com/doi/10.1002/ps.3566/full
Funding source: This project is funded by the Citrus Research and Development Foundation.