The snapshots on this page describe tools that are currently being investigated to manage the psyllid and HLB or to facilitate research with these goals.
-
What makes lemons, oranges and limes look and taste different?
Written by Drs. Peggy Lemaux (UC Berkeley) and Elizabeth Grafton-Cardwell (UC Riverside)
Revised July 1, 2025.Living organisms, including plants, have many cells. Each cell has the genetic information to dictate the way the plant looks, tastes and feels. It also determines whether plants can be infected by pathogens that cause diseases, like Huanglongbing (HLB) also known as citrus greening.
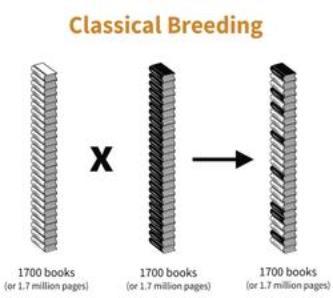
The genetic information is contained in long strings of chemicals, called DNA. The DNA is organized into individual units, called genes that specify traits, like a lemon’s tart taste or a tangerine’s orange color. The genetic information, or genome, could be thought of as a collection of books with information on many different topics. Each organism has its own set of books and pages. While some information is similar among different organisms, some is altered - making lemons look and taste different from oranges, limes and mandarins.
If alphabetic letters were used to represent each unit in the long string of chemicals making up the genetic information of citrus, it would require about 35 books, each of 1000 pages, to contain all of the information in the cell of a citrus tree. Identity of the chemical units in sweet orange has been determined, showing that there are nearly 25,000 genes specifying its traits.
How is classical breeding used to make new citrus varieties?
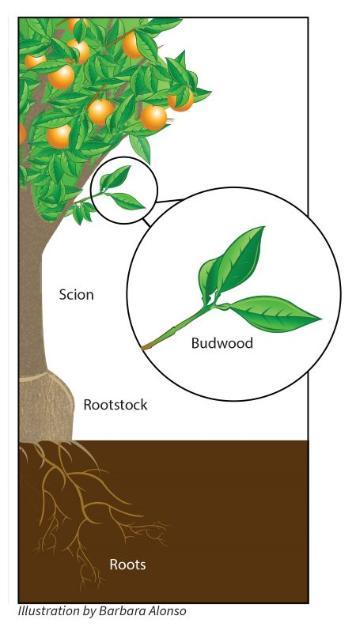
 Most plants consist of roots, stems and leaves joined together from the time they germinate from the seed. But citrus, and many other tree crops, are made of two parts that are grafted together to form the mature tree. A rootstock forms the part of the tree that is mostly below ground, and the scion is responsible for the upper part of the tree: trunk, branches, leaves and, most importantly, fruit.
Most plants consist of roots, stems and leaves joined together from the time they germinate from the seed. But citrus, and many other tree crops, are made of two parts that are grafted together to form the mature tree. A rootstock forms the part of the tree that is mostly below ground, and the scion is responsible for the upper part of the tree: trunk, branches, leaves and, most importantly, fruit. One way breeders create better citrus trees involves selecting for different kinds of improvements in the rootstock and the scion portions of the tree. In rootstock varieties, they look for tree size, yield, disease and insect tolerance, soil adaptation, freeze tolerance, fruit quality and compatibility with the scion. In the scion varieties they look for fruit size and flavor, rind and flesh color, rind thickness, levels of acids sugars and numbers of seeds.
What if a breeder wants to create a better rootstock? One way is for the breeder to cross two citrus trees to create a new tree with improved traits. But what happens to the 35 books from each parent? Does the new tree have 70 books of information? No. Genetic rules dictate that the resulting tree can only have about 35 books. This results from randomly keeping about half of the information from one parent and half from the other. Each new tree has unique combinations of genes and traits.
Crosses are made by taking pollen (male cells), from one tree and delivering them via a pollen tube to the egg (female cell) of another compatible tree. The male and female cells come together and ultimately become seeds that create the next generation of trees. Breeders observe the resulting trees, choosing those with desired traits. Seeds resulting from classical breeding yield trees with modified genetic information containing new mixes of genes and traits. Examples in citrus from such breeding efforts are Minneola tangelo and Gold Nugget and Pixie mandarins. It is now known that many common citrus varietal types, such as oranges, lemons, and limes, originated in ancient times by such crosses.
Other methods for creating new citrus varieties
While crossing two citrus varieties can give rise to new trees, there are other ways that can be used to change traits. Sometimes changes in the order or nature of the chemical units occur naturally from the effects of sunlight. This can result in a mutation, or genetic change, that people might notice in a part of the tree. An example is the navel orange, Cara Cara. Its pink flesh resulted from a mutation in a Washington navel orange tree. Irradiating citrus budwood can also induce these genetic changes. The irradiated budwood is grafted to a rootstock and grown for a period of time and if a new trait is observed, budwood from that tree can become a new variety of citrus. Examples of varieties created by irradiating buds are the low-seeded mandarins Tango, Daisy SL, Fairchild LS and Kinnow LS.
More recently breeders have used a process, called marker-assisted selection (MAS). This approach involves creation of a “table of contents” that identifies locations for genes specifying certain traits. MAS is like using the “find” command in a word processing system to identify particular sentences in a book. By knowing phrases in a sentence, text can be found easily in the book. In plant breeding, breeders use the naturally occurring chemical tags in DNA to identify specific genes. If chemical tags are lacking, the plant does not have a particular trait and breeders don’t have to wait for the plant to mature to eliminate it from the selection process. In citrus, MAS is used on a very limited basis – more for rootstocks than scions – but, as more information on tags that predict traits becomes available, it will be more widely used.
Creation of a new citrus variety by crossing two varieties, whether MAS is used or not, or by the existence or creation of mutations, whether natural or induced by radiation, results in a plant and a fruit with modified genetic information. Exactly the same process happens when two humans have a child that then has genetic information that is different from each parent.
What is a genetically engineered organism or GMO?
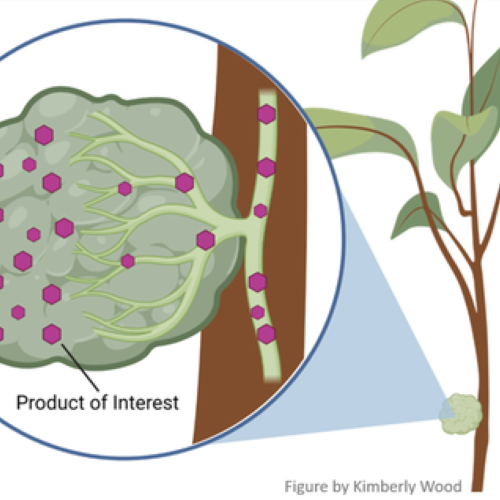
What if another citrus variety or another vegetable, like spinach, had a trait that would protect the citrus tree from diseases, like HLB? It turns out that the DNA “language” in in all organisms is the same, so genetic information from any organism, such as spinach, can be transferred to citrus and be used to make a new trait.
How is this done? Once certain information is known about the trait, for example a gene in spinach protects citrus against HLB, that genetic information can
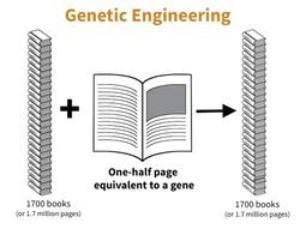 be introduced into the tree using a process somewhat like that in a word processing system. Once found, chemical scissors are used to remove the information, and chemical paste is used to place the information into the genetic information of another plant. The new information, equal to a half-page, will be passed on to the next generation. This process, termed genetic engineering, gives rise to plants with modified genetic information – termed GMO’s (genetically modified organisms) by the popular press.
be introduced into the tree using a process somewhat like that in a word processing system. Once found, chemical scissors are used to remove the information, and chemical paste is used to place the information into the genetic information of another plant. The new information, equal to a half-page, will be passed on to the next generation. This process, termed genetic engineering, gives rise to plants with modified genetic information – termed GMO’s (genetically modified organisms) by the popular press.In citrus, the process of genetic engineering has been used experimentally to protect against citrus tristeza virus (CTV) and canker diseases, provide resistance to certain insects, create dwarf varieties and afford drought and salt tolerance. Some of these efforts use genetic information from non-citrus organisms. More recently, efforts have focused on using genes from other citrus varieties to lower acidity, create blood orange color, increase disease resistance and lower levels of the chemical in grapefruit that interferes with statin drugs.
Are genetically engineered (GMO) crops and foods on the market?
While genetic engineering has only been used experimentally in citrus, there are six major GE crop plants in the U.S. that are being grown commercially: alfalfa, canola, corn, cotton, soybean and sugar beet. Most U.S. acreage of these crops is planted in engineered varieties.
Two traits are commonly introduced into GE crops. First, insect tolerance is achieved by introducing a gene from a naturally occurring bacterium, Bacillus thuringiensis. This gene codes for a toxin that binds to and destroys specific cells in the guts of particular pest insects. Second, herbicide tolerance, allows engineered plants to survive when that herbicide is sprayed, while weeds are killed.
There is a common misperception among consumers that most foods are genetically engineered. This is because numerous minor ingredients from corn, canola and soybean, like cornstarch, canola oil and soy lecithin, are present in processed foods. But only three engineered whole fruits or vegetables are in the commercial market today, papaya, certain kinds of summer squash and sweet corn. Papaya and squash were engineered to resist destructive viruses. In 2015, FDA approved engineered potatoes with reduced bruising and lower starch and GE apples with reduced browning. These products may be on the market in the future.
Have genetically engineered insects been released?
The same process used to engineer plants can be used to engineer insects, like the Asian Citrus Psyllid (ACP) that carries the bacterium that causes HLB to kill citrus trees. Is it possible to engineer ACP so that it is no longer ‘hospitable’ to the HLB bacterium? Researchers with the USDA-sponsored NuPsyllid project are trying several ways to engineer the ACP insect so it can not host the bacterium and so no longer transmits the disease. It will take a number of years to see if such strategies are successful in curbing the spread of HLB.
Funding source: This project is funded by the USDA-NIFA and Citrus Research and Development Foundation.
-
Novel tools for antimicrobial testing and discovery of new HLB therapies
Research by Kranthi Mandadi, Texas A&M University
Article written by Kranthi Mandadi, Sonia Irigoyen, Manikandan Ramasamy, Peggy G. Lemaux, Lukasz Stelinski, Madison Sankovitz, Monique Rivera
Revised July 1, 2025.What is the technique?
A consensus study by the U.S. National Academy of Sciences on citrus greening research development concluded that there is no single effective therapy to fight the disease and suggested that a combination of therapies and management practices will likely be most successful in managing the disease. To discover new anti-CLas therapies, we developed an innovative CLas-citrus hairy root system that enables high throughput screening and discovery of antimicrobial peptides, small molecules, defense-related genes, and gene editing (CRISPR) targets for HLB management1.
What are the challenges and opportunities?
Challenges in finding new HLB therapies include the inability to grow the causal pathogen of HLB, Candidatus Liberibacter asiaticus (CLas), and the long developmental period of perennial citrus trees. Standard antimicrobial testing relies on whole plant assays and laborious and time-consuming plant transformations - not suited for screening large numbers of samples. Some researchers have used dista
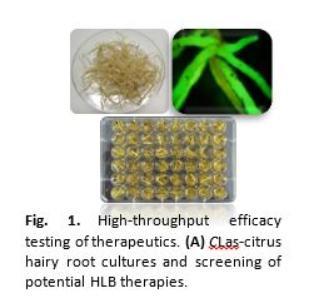 ntly related microbes and annual plants as substitutes. However, results obtained from such substitutes may not represent the situation with CLas-infected citrus due to differences in disease expression and severity. We recently showed that citrus shoots can be triggered to produce hairy roots (Figure 1)1. Because the transport vessels in hairy roots mimic the natural environment of CLas, they support the growth of the bacterium. We showed that citrus hairy roots can be used for high throughput screening of potential new HLB therapies. A key advantage to hairy root assays is they can be performed ~3-4 times faster than whole plant assays - critical for accelerating the discovery of therapies. Using this system, we screened candidates and discovered new antimicrobial peptides, immune regulators, and small molecules, which inhibited CLas in citrus root tissues. One or more of these candidates, currently being evaluated in plants, might be developed for HLB management.
ntly related microbes and annual plants as substitutes. However, results obtained from such substitutes may not represent the situation with CLas-infected citrus due to differences in disease expression and severity. We recently showed that citrus shoots can be triggered to produce hairy roots (Figure 1)1. Because the transport vessels in hairy roots mimic the natural environment of CLas, they support the growth of the bacterium. We showed that citrus hairy roots can be used for high throughput screening of potential new HLB therapies. A key advantage to hairy root assays is they can be performed ~3-4 times faster than whole plant assays - critical for accelerating the discovery of therapies. Using this system, we screened candidates and discovered new antimicrobial peptides, immune regulators, and small molecules, which inhibited CLas in citrus root tissues. One or more of these candidates, currently being evaluated in plants, might be developed for HLB management.
Additionally, we have extended the technology to multiple public- and private-sector collaborators and stakeholders to maximize the impact of these discoveries and to speed deployment of new HLB control measures.Who is working on this project?
Kranthi Mandadi, Sonia Irigoyen, Mani Ramasamy, San Fernando (Texas A&M AgriLife/Texas A&M University); Veronica Ancona, Mamoudou Setamou (Texas A&M-Kingsville); James Borneman (University of California, Riverside); William Dawson, Choaa El-Mohtar (University of Florida); Mike Irey (Southern Gardens Citrus).
References
1 Irigoyen, S. et al. Plant hairy roots enable high throughput identification of new antimicrobials against Candidatus Liberibacter spp. Nature Commun. 11, 5802 (2020).
Please see related:
Hairy root matrix used to culture CLas to rapidly screen antimicrobials for improved HLB management
Dr. Kranthi Mandadi, Texas A&M University.Funding: USDA NIFA, FFAR, Southern Gardens Citrus and Texas A&M AgriLife Research
-
Living with HLB - area-wide integrated management system for ACP in Texas
Research by Mamoudou Sétamou, Texas A&M University-Kingsville Citrus Center
Article written by Sara Garcia Figuera and Mamoudou Sétamou. Edited by Monique Rivera, Peggy G. Lemaux.
Revised July 1, 2025.What is the technique?
Texas is the third largest citrus-producing state in the US, with 65% of its citrus acreage focused on red grapefruit production for the fresh market. Most commercial citrus groves in Texas are located in the southern end of the Lower Rio Grande Valley, where the Asian citrus psyllid (ACP) was first detected in 2001 and the HLB bacterium in 2012. Since then, both have spread throughout the region and are now established, putting Texas citrus production under grave threat.
 The area-wide integrated management system (AIMS) was developed for Texas citrus growers to control ACP populations on a landscape scale and maintain citrus production at a profitable level, even with widespread HLB. AIMS consists of three coordinated area-wide insecticide sprays (pyrethroids, organophosphates, neonicotinoids in conventional groves and biopesticides including mineral and plant oils, pyrethrins and/or kaolin in organic groves), two during the dormant period, in November and February, and one in mid/late August, before the September rains that trigger profuse flush (new leaf growth) production. Scouts, hired by the Texas Citrus Pest and Disease Management Corporation, call the growers in each of the two management zones to let them know when it is time for the coordinated dormant sprays, and organic growers are asked to spray twice for each conventional spray, at the beginning and at the end of each spray window. Additional sprays are recommended in individual properties at the onset of each flush cycle during the active growing season, from April to September. To time these individual sprays at the most effective moment, growers need to monitor the ACP populations and flush on the grove borders. When there is flush, it is as if citrus trees had turned on a green light for the ACP, so it is crucial to spray right when ACP adults are present and start reproducing. These sprays can be combined with pesticides targeting other pests and diseases, thus the concept of integrated management.
The area-wide integrated management system (AIMS) was developed for Texas citrus growers to control ACP populations on a landscape scale and maintain citrus production at a profitable level, even with widespread HLB. AIMS consists of three coordinated area-wide insecticide sprays (pyrethroids, organophosphates, neonicotinoids in conventional groves and biopesticides including mineral and plant oils, pyrethrins and/or kaolin in organic groves), two during the dormant period, in November and February, and one in mid/late August, before the September rains that trigger profuse flush (new leaf growth) production. Scouts, hired by the Texas Citrus Pest and Disease Management Corporation, call the growers in each of the two management zones to let them know when it is time for the coordinated dormant sprays, and organic growers are asked to spray twice for each conventional spray, at the beginning and at the end of each spray window. Additional sprays are recommended in individual properties at the onset of each flush cycle during the active growing season, from April to September. To time these individual sprays at the most effective moment, growers need to monitor the ACP populations and flush on the grove borders. When there is flush, it is as if citrus trees had turned on a green light for the ACP, so it is crucial to spray right when ACP adults are present and start reproducing. These sprays can be combined with pesticides targeting other pests and diseases, thus the concept of integrated management.How does it improve HLB management?
By targeting the ACP population on an area-wide scale, focusing on several individual properties at the same time, citrus growers can minimize ACP movement between groves and prevent transmission of the HLB bacterium. The two main objectives of AIMS are: (1) to reduce ACP populations during the overwintering period, while citrus trees are dormant, so that populations are at their lowest level when they start reproducing at the onset of citrus growth and; (2) to prevent ACP reproduction on citrus flush. ACP inject the HLB bacterium when they feed, and they exclusively lay their eggs on citrus flush. When the eggs hatch
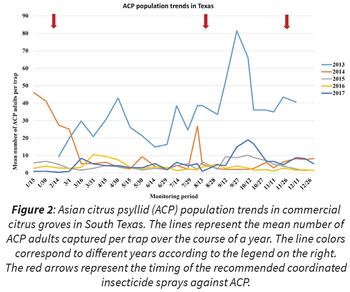 , nymphs feed on infected flush and acquire the bacterium, transmitting it to other trees when they become adults and fly away. Therefore, coordinated sprays lower the ACP population at the end of the fall and before the spring flush, and individual sprays timed before each flush cycle prevent ACP reproduction on flush, reducing HLB transmission. AIMS has been successful at keeping the ACP populations low and maintaining citrus production in Texas at a profitable level. Indeed, a gradual year-to-year reduction of ACP populations industry-wide has been observed since the inception of this program ten years ago in January 2010.
, nymphs feed on infected flush and acquire the bacterium, transmitting it to other trees when they become adults and fly away. Therefore, coordinated sprays lower the ACP population at the end of the fall and before the spring flush, and individual sprays timed before each flush cycle prevent ACP reproduction on flush, reducing HLB transmission. AIMS has been successful at keeping the ACP populations low and maintaining citrus production in Texas at a profitable level. Indeed, a gradual year-to-year reduction of ACP populations industry-wide has been observed since the inception of this program ten years ago in January 2010.Who is working on the project?
Dr. Mamoudou Sétamou, Professor of Entomology at Texas A&M University-Kingsville Citrus Center, developed AIMS in close collaboration with Drs. David Bartels and Matt Ciomperlik scientists at APHIS-PPQ in Mission, Texas, and the Texas citrus industry and continues to work with the industry to develop new tools and refine existing approaches.
What are the challenges and opportunities?
In the Lower Rio Grande Valley, commercial citrus groves are interspersed with residential neighborhoods with abundant backyard citrus trees, so there is a constant movement of ACP from backyards to groves, and vice versa. This poses a significant challenge when trying to manage ACP at a landscape scale, because homeowners are not used to controlling for pests, and most are not willing to remove their valued citrus trees in order to prevent infection with HLB. Because of this, the biocontrol agent, Tamarixia radiata has been released in residential neighborhoods to reduce ACP populations. Despite the influx of ACP from residential citrus, AIMS has been successful at maintaining ACP populations at a low level. This strategy was developed building on experiences learned from Florida and adapting them to Texas conditions. In turn, because Texas is at a more advanced state of the HLB epidemic than California, there is an opportunity for California to learn from the experiences acquired in Texas to optimize the area-wide management program that has been implemented in Southern California.
Funding source: We express our sincere gratitude to USDA APHIS-PPQ for funding through the Citrus Commodity Pest Survey Cooperative Agreements.
-
Studying Liberibacter crescens to learn more about the causal agent of huanglongbing
Research by Drs. Marta Sena-Vélez and Kathryn Jones, Florida State University
Article written by Marta Sena-Vélez. Edited by Sara Garcia-Figuera and Peggy G. Lemaux.
Revised July 1, 2025.What is the problem?
Studying how a pathogen interacts with its plant host and its insect vector is of great importance for understanding disease development and transmission in order to prevent and control plant diseases. Many of these studies are performed under greenhouse and field conditions; however, such investigations also require laboratory studies of the organism in 'pure culture', in the absence of other microorganisms, and under controlled conditions. Without these controlled experiments, it is impossible to study a microbe’s growth requirements or its pathogenic weapons, also known as virulence factors. To do this, it requires mimicking the natural environments in which each specific bacterium lives and reproduces in the lab.
The main hurdle in studying the huanglongbing bacterium, "Candidatus Liberibacter asiaticus" (CLas), and its interactions with its host citrus species and insect vector, the Asian citrus psyllid, is the lack of a medium that supports its growth in pure culture. CLas lives in citrus phloem and has adapted very well to this environment. Because of this, it cannot produce most metabolites required for its own development. Thus, CLas must acquire them from outside--in the same way plants need water and nutrients provided to them by humans. Despite multiple attempts to recreate a medium that replicates the conditions in the phloem or in specific psyllid tissues, the complexity of CLas niches, as well as our limited understanding of CLas metabolic pathways, has prevented being able to culture this pathogen.
What is the technique?
Worldwide efforts are being made by researchers to grow CLas, including the use of surrogates of bacteria that are closely related to CLas. The best model to date is Liberibacter crescens (Figure 1), the only culturable member of the group of bacteria to which CLas belongs. This bacterium, first isolated in 1995 from a diseased mountain papaya tree in Puerto Rico, is not pathogenic and is not transmitted by any known vector. Thus, no quarantine measures are needed to work with it in HLB-free regions. L. crescens shares 94% of the genetic information (DNA) of CLas, and the similarity of the genes required for important metabolic pathways can help identify which factors may affect the inability to culture CLas. In addition, L. crescens can be genetically modified to study the function of genes shared with CLas, or to produce CLas proteins. Because of its similarity, L. crescens is the best bacterial model for studying huanglongbing and other diseases caused by Liberibacter species, like zebra chip disease of potato.
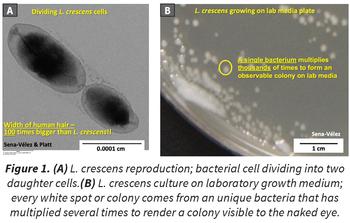
Despite its being the best CLas surrogate, L. crescens is still challenging to grow. In our studies, we greatly improved L. crescens’ growth rate and survival in the laboratory by altering multiple culture conditions and developing new growth medium formulations. Furthermore, we showed that L. crescens consumes organic acids rapidly, creating poisonously alkaline conditions in the growth medium that impede the ability of L. crescens to reproduce, and therefore its ability to be cultured (Figure 2). In addition, our results were used to build a mathematical model of L. crescens growth, based on bacterial stress and adaptation to deleterious conditions. This will help better understand L. crescens growth and predict conditions that will help in designing new growth medium as well as a first approach to understand why CLas can’t be cultured. This model fits the L. crescens culture data, but more importantly was used to make predictions that have been confirmed experimentally, e.g., culturability loss is reduced when the pH is corrected by organic acid addition the day the culture reaches pH 8.5 (day 5 of growth in our conditions), but not a day after showing that alkaline stress is crucial for L. crescens culturability.
Who is working on the project?
Dr. Marta Sena-Vélez, Dr. Kathryn Jones, Sean Holland and Darryl Trickey at Florida State University, Department of Biology, and Drs. Dean Gabriel and Mukesh Jain at the University of Florida, Department of Plant Pathology, are working on culturing L. crescens and CLas. Drs. Manu Aggarwal and Nick Cogan at FSU, Department of Mathematics, are working on mathematical modeling of L. crescens growth and metabolism.
What are the challenges and opportunities?
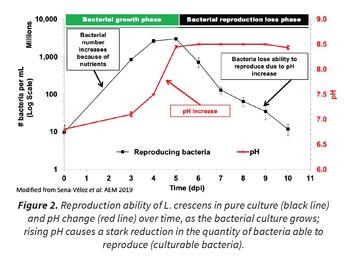
Similarity in core metabolic pathways for carbon and nitrogen utilization between L. crescens and CLas means that work on culture requirements of L. crescens provides valuable information for the development of a medium that can be used to grow CLas. Our finding that L. crescens creates alkaline conditions in its medium (Figure 2 red line), such that it poisons itself, is unusual for a bacterium. Determining if such alkali production plays a role in the failure of CLas to grow in culture is one avenue for investigation opened up by our research on L. crescens. Further approaches include investigation of other biochemical pathways related to the utilization of different nutrient sources in both L. crescens and CLas. Improved growth of L. crescens in the laboratory will make it easier to use this model to study CLas proteins important for communication with the environment and gene regulation networks and to better comprehend CLas behavior in both nature and the laboratory. An improved understanding of the basic biology of CLas will help us understand how to fight it. Use of L. crescens as a culturable surrogate for CLas has allowed us to gain a foothold in understanding Liberibacter biology and will permit the development of new tools to further understand and control the devastating huanglongbing disease of citrus.
Funding source: This work was funded by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2014-67013-21579 to K.M.J., USDA NIFA SCRI Citrus Disease Research and Extension (CDRE) award 2016-70016-24844 to D.H.G. and subaward UFDSP00011165 to K.M.J
-
Using particle films to manage AC
Research by Dr. Christopher Vincent, University of Florida
Article written by Christopher Vincent, Monique Rivera, Elizabeth Grafton-Cardwell, & Peggy G. Lemaux.
Revised July 10, 2025.What is the technique?
Particle films are fine particles that are applied to plants to form a thin residue, or “film,” over the leaf surface. These films reflect light, giving the plant a bright or white appearance. Usually they are sprayed in a water suspension through a standard sprayer. The most common type of particle film is made from natural kaolin clay, a soft, white aluminum silicate, that is mined and filtered for a small particl
 e size. Another type of film consists of diatomaceous earth. The particles are applied to crops to manage environmental challenges, like high heat or sunburn, or manage pests, such as aphids. Particle films enhance plant growth under most conditions by helping the plants achieve a more optimal light distribution. The particles reflect light away from the outer leaves which keeps them from experiencing high light stress which, in turn, helps to reduce water loss and keep leaf temperatures within an optimal range. Some of the light reflected by the particle film appears to be redistributed deeper into the canopy which would assist more of the tree’s leaves to have access to light. Particle films reduce infestations by some pests because the particle films hide the natural plant colors that help some insects find their plant host. Recently we began field-testing mixes that use a particle film (Surround WP), made from kaolin that has an added red dye to improve the effect on pest management. Red enhances the pest management effect by reducing reflectance of yellow, blue, and ultraviolet light, which attract Asian citrus psyllid (ACP), the vector of HLB (Figure 1).
e size. Another type of film consists of diatomaceous earth. The particles are applied to crops to manage environmental challenges, like high heat or sunburn, or manage pests, such as aphids. Particle films enhance plant growth under most conditions by helping the plants achieve a more optimal light distribution. The particles reflect light away from the outer leaves which keeps them from experiencing high light stress which, in turn, helps to reduce water loss and keep leaf temperatures within an optimal range. Some of the light reflected by the particle film appears to be redistributed deeper into the canopy which would assist more of the tree’s leaves to have access to light. Particle films reduce infestations by some pests because the particle films hide the natural plant colors that help some insects find their plant host. Recently we began field-testing mixes that use a particle film (Surround WP), made from kaolin that has an added red dye to improve the effect on pest management. Red enhances the pest management effect by reducing reflectance of yellow, blue, and ultraviolet light, which attract Asian citrus psyllid (ACP), the vector of HLB (Figure 1).How are particle films used to manage HLB?
Particle films can dramatically r
 educe arrival of ACP. In our experimental plots in Florida, under high disease and ACP pressure, both natural white and red-dyed kaolin reduced ACP populations by more than 80% as compared to monthly insecticide treatments. Red kaolin produces a small reduction (4-5%) in ACP populations, relative to the white. Both treatments delayed HLB infection; the first detected infection in the red treatment was nearly 1 year after the first detected infections in the untreated trees. Both treatments have had positive effects on young tree growth, with kaolin-treated tree trunks having a nearly doubled girth versus untreated trees and much larger and denser canopies. Several growers are using kaolin regularly and report positive results in terms of tree health and growth (Figure 2).
educe arrival of ACP. In our experimental plots in Florida, under high disease and ACP pressure, both natural white and red-dyed kaolin reduced ACP populations by more than 80% as compared to monthly insecticide treatments. Red kaolin produces a small reduction (4-5%) in ACP populations, relative to the white. Both treatments delayed HLB infection; the first detected infection in the red treatment was nearly 1 year after the first detected infections in the untreated trees. Both treatments have had positive effects on young tree growth, with kaolin-treated tree trunks having a nearly doubled girth versus untreated trees and much larger and denser canopies. Several growers are using kaolin regularly and report positive results in terms of tree health and growth (Figure 2).Who is working on this project?
This project is being conducted by several people in the Tree Ecophysiology Laboratory at the University of Florida Citrus Research and Education Center, with help from Ed Etxeberria and Pedro Gonzalez at the same center. Additionally, a number of growers are experimenting with ways to apply kaolin more effectively.
What are the challenges and opportunities?
The major challenge in Florida is the ability to withstand rainfall. Frequent rains tend to wash off particle films, which are only effective as long as they cover the entire leaf. To maintain constant coverage in Florida we have had to apply every two weeks on average, and growers have noted similar frequencies, which drives the cost up. In California, growers use this product for sunburn protection and have commented that in desert conditions, they have difficulty adhering the product to new foliage. For these reasons, research is needed to develop formulations that adhere to the leaves better. Additionally, these films have disrupted natural enemies and led to California red scale outbreaks. To minimize this problem, we intend to test the efficacy of border treatments where the psyllids are most commonly found. Major opportunities are that particle films are appropriate for both organic or conventional use, and that they can effectively decrease ACP and delay HLB infection, while simultaneously enhancing growth.
Funding source: This project is funded by the Citrus Research and Development Foundation.
-
Hairy root matrix used to culture CLas to rapidly screen antimicrobials for improved HLB management
Research by Dr. Kranthi Mandadi, Texas A&M University
Article written by Kranthi Mandadi, Veronica Ancona, Steve Futch, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, Lukasz Stelinski, Monique Rivera & Sara Garcia-Figuera.
Revised July 10, 2025.Click here to download PDF
What is the technique?Most plant pathogens can be studied in the laboratory. The ability to keep pathogens in petri dishes or test tubes containing nutrients in the laboratory allows the pathogen to be easily manipulated and studied. A major difficulty in studying Candidatus Liberibacter asiaticus (CLas), the
 bacterium thought to cause huanglongbing (HLB), has been that the bacterium can not be grown using these traditional laboratory methods. The inability to culture the HLB bacterium, CLas, in the lab makes it very difficult to develop cures for the disease. Our research team has developed a novel technique for laboratory cultivation of CLas, using so-called hairy roots, which are plant tissues that mimic a microbe’s natural environment. Hairy root cultures support CLas bacterial growth and these cultures can be used to screen antimicrobials to find compounds that control and/or suppress CLas. Once discovered, antimicrobials could be applied to the foliage or injected into the trees to prevent establishment or reduce the impact of HLB in citrus trees. The beauty of the hairy root method is that it allows researchers to rapidly screen many antimicrobials (high throughput) and urgency is necessary in combating this deadly disease.
bacterium thought to cause huanglongbing (HLB), has been that the bacterium can not be grown using these traditional laboratory methods. The inability to culture the HLB bacterium, CLas, in the lab makes it very difficult to develop cures for the disease. Our research team has developed a novel technique for laboratory cultivation of CLas, using so-called hairy roots, which are plant tissues that mimic a microbe’s natural environment. Hairy root cultures support CLas bacterial growth and these cultures can be used to screen antimicrobials to find compounds that control and/or suppress CLas. Once discovered, antimicrobials could be applied to the foliage or injected into the trees to prevent establishment or reduce the impact of HLB in citrus trees. The beauty of the hairy root method is that it allows researchers to rapidly screen many antimicrobials (high throughput) and urgency is necessary in combating this deadly disease.What are the challenges and opportunities?
Currently, several antimicrobial compounds are being tested against HLB, including bactericides (antibiotics) and compounds that stimulate tree defense systems. To evaluate efficacy, the conventional, time-consuming approach is to perform foliar (leaf) sprays, trunk injections, or soil drenching, using the antibiotic, or to screen against related pathogens, such as Liberibacter cresens, that can be minimally cultured in the laboratory. However, the effectiveness of these approaches can be inconsistent and costly, especially when trying to work with mature trees, limiting the ability of researchers to screen the vast number of chemistries that exist. Our proposed CLas culturing in hairy roots and screening pipeline protocol will allow evaluation of new antimicrobial compounds (e.g., small molecules, disease resistance and susceptibility genes/proteins) against CLas much faster (higher throughput) and at a lower cost, thus saving a significant amount of resources and time. Hastening implementation of a possible successful treatment and/or therapy is critical to solving the HLB problem for the citrus industry.
Who is working on this project?
Kranthi Mandadi, Assistant Professor, Plant Pathology and Microbiology, Texas A&M AgriLife Research & Extension Center, Weslaco; San Fernando, Professor, Biological and Agricultural Engineering, Texas A&M University; Veronica Ancona, Assistant Professor, Texas A&M-Kingsville Citrus Center; Gitta Coaker, Professor, University of California (UC), Davis; Elizabeth Grafton-Cardwell, Research Entomologist, UC Riverside and Director of Lindcove Research and Extension Center; William Dawson, Professor, University of Florida (UFl) Citrus Center; Stephen Futch, Extension Agent IV, UFl Citrus Research and Education Center; Mike Irey, Director of Research and Business Development, Southern Gardens Citrus, Florida.
Funding source: This study was supported by funds fromFoundation for Food and Agricultural Research, Southern Gardens Citrus, USDA-NIFA-CDRE and Texas A&M AgriLife Research Insect-Vectored Diseases Seed Grant.
-
Attractants and traps for ACP management
Research by Dr. Lukasz Stelinski, University of Florida
Article written by Lukasz Stelinski, Elizabeth Grafton-Cardwell and Peggy G. Lemaux.
Revised July 10, 2025.What is the technique?
Attractants for insect pests of agricultural crops play an increasingly important role in management programs. Chemical, visual, and auditory cues that attract insects are identified during laboratory investigations and then verified for their attractiveness in the field. These cues are then exploited to serve as lures deployed with traps to attract pests coming from potentially long distances. This allows detection and monitoring of target pests and/or attracting and killing them t
 o reduce their numbers in the orchard.
o reduce their numbers in the orchard.
Traps may be coated with a sticky substance to ensnare individuals (Fig. 1A) or the insect may be trapped within an enclosure containing a killing agent (Fig. 1 B). Such traps are optimized for pests of economic significance by taking advantage of the chemical and visual cues associated with the resources insects search for, such as host plants for egg laying or mates for reproduction.Asian citrus psyllids (ACP) use multiple sensory modalities [vision, olfaction, contact chemoreception, gustation (taste), perception of auditory or vibrational stimuli] to locate host plants to feed on and reproduce. ACP are attracted to wavelengths perceived as yellow or lime-green in the human visual spectrum. UV wavelengths further increase ACP attraction to visual cues. An effective scent attractant blend can contribute to the attraction of ACP to visually attractive traps (Patt and Setamou 2010). After landing, ACP taste their potential food source and determine if the plant is suitable when they first insert their mouthparts into the plant. A specific blend of chemicals was identified that elicits a feeding response from ACP upon contact with an attractive substance (Lapointe et al. 2016). Currently available traps for monitoring ACP consist of flat, yellow-color panels (approx. 20 x 15 cm) coated with adhesive to ensnare encountering insects (Fig. 1A). In some cases, panels are also sold with proprietary blends of volatiles that purport to increase catch of ACP.
How does a better trap improve HLB management?
Trap devices for ACP are intended for: 1) detecting infestations in new areas; 2) triggering insecticide treatments to maintain psyllids below a threshold; and 3) timing insecticide sprays to have maximum impact on populations. Attractants for ACP can also be combined with a low-dosage killing agent into a single device that can be deployed for direct population reductions. Psyllids spread huanglongbing bacteria, and so reducing their numbers is a critical component of reducing impact of the disease.
Who is working on the Project?
Drs. Mamoudou Setamou (Texas A&M University), Steven Lapointe, David Hall, Joseph Patt (USDA-ARS-Fort Pierce, FL), Justin George, Xavier Martini, and Lukasz Stelinski (University of Florida), have collaborated extensively and also lead individual research efforts on identification of ACP attractants and development of practical ACP traps and lures.
What are the challenges?
The search for effective chemical attractants has yielded only weakly effective lures that enhance ACP adult orientation to visually attractive traps by only 2-2.5 fold. Currently available traps attract ACP from relatively short distances of only 6-30 feet, after ACP have already infested host plant foliage. The visual and olfactory cues of natural citrus foliage, particularly in a crop monoculture, far outcompete traps; they are simply much more attractive than foliage-mimicking traps. Thus, they are not very useful for drawing psyllids away from citrus.
In addition, such traps are not always reliable predictors of population outbreaks.
Efforts continue to refine chemical lures to improve their effectiveness under varying seasonal conditions and within diverse citrus genotypes grown in different regions. Furthermore, improvements continue on integrating several attractive cues (color, smell, and taste) with a toxicant to develop attract-&-kill stations that may alleviate the need for repeated insecticide spray applications for ACP management.
Citations:
Lapointe, S.L., George, J., and D. G. Hall. 2016. A phagostimulant blend for the Asian citrus psyllid. J. Chem. Ecol. 42: 941-951.Patt, J.M., Sétamou, M. 2010. Responses of the Asian citrus psyllid to volatiles emitted by the flushing shoots of its rutaceous host plants. Environ Entomol 39: 618–624.
Funding source: This project is funded by the USDA Multiagency Coordinated Funds.
-
Growing citrus under enclosures
Research by Dr. Rhuanito Soranz Ferrarezi, University of Florida
Article written by Rhuanito Soranz Ferrarezi, Elizabeth Grafton-Cardwell, Peggy G. Lemaux, & Lukasz Stelinski.
Revised July 10, 2025.Click here to download PDF
What is the technique?Citrus production under protected environments reduces huanglongbing (HLB) disease incidence and damage by excluding the Asian citrus psyllid (ACP) vector from access to citrus trees by surrounding them with a screen barrier. In addition to protecting citrus from the disease, it greatly reduces insecticidal sprays applied to control psyllids. This reduces costs and selection for insecticide resistance in the psyllid.
How does it improve HLB management?

Completely enclosed screenhouses physically exclude the ACP and thus prevent inoculation of the plants with the CLas bacteria that causes HLB. A screened enclosure in Florida fully protected young grapefruit (Citrus × paradisi) trees from eggs, nymphs and adult ACP and no trees tested positive for CLas inside the enclosure after two years of monitoring. In contrast 100% of surveyed trees in the nearby open-air plots tested positive for CLas during the same period. Thus, the use of screenhouses offered a substantial level of protection against the establishment of HLB, compared to management programs founded solely upon protecting the citrus from ACP using insecticidal sprays.
Who is working on the Project?
The citrus under protective screen system was developed at the University of Florida/Institute of Food and Agricultural Sciences (UF/IFAS) Indian River Research and Education Center in Fort Pierce, FL and has been tested at the UF/IFAS Citrus Research and Education Center in Lake Alfred, FL. The economics of citrus under protective screens (CUPS) is being determined. To date, there are 50 acres of commercial CUPS with three growers in Florida, and at least 150 more acres are planned (Eduardo Pines and Steven Callaham, personal communication).
Drs. Rhuanito Ferrarezi, Arnold Schumann, Ariel Singerman, Jawwad Qureshi, Philippe Rolshausen (UC Davis), Andrew Krajewski (International Citrus Technologies, Australia), Elizabeth Grafton-Cardwell, Stephen Futch, Chris Oswalt and Garima Kakkar are currently part of this research.
Dr. Ferrarezi is with the University of Florida Horticultural Sciences Department He is a professor of citrus horticulture and is located at the Indian River Research and Education Center in Fort Pierce, FL. All other researchers with the exceptions of Grafton-Cardwell (UC Riverside), Krajewski (International Citrus Technologies, Australia), and Rolshausen (UC Davis) are at the University of Florida.
What are the challenges and opportunities?
Researchers are still answering many questions about the technology, such as the most suitable citrus varieties, the installation and maintenance costs, the economics and level of fruit production, the level of labor needed, pest and disease control, resistance to extreme weather conditions such as frost, tropical storms and hurricanes, irrigation and fertilization, canopy management and other technical aspects. The Florida CUPS group recently received a USDA-NIFA-CDRE grant to address a number of these topics.
Ferrarezi RS, Wright AL, Boman BJ, Schumann AW, Gmitter FG, Grosser JW. 2017. Protected fresh grapefruit cultivation systems: Antipsyllid screen effects on environmental variables inside enclosures. HortTechnology 27(5): 675-681.
http://dx.doi.org/10.21273/HORTTECH03790-17Ferrarezi RS, Wright AL, Boman BJ, Schumann AW, Gmitter FG, Grosser JW. 2017. Protected fresh grapefruit cultivation systems: Antipsyllid screen effects on plant growth and leaf transpiration, vapor pressure de?cit, and nutrition.
HortTechnology 27(5): 666-674. http://dx.doi.org/10.21273/HORTTECH03789-17
Funding source: This project is funded by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2018-70016-27387.